|
 |
- Search
AbstractObjectivesTo evaluate the significance of laryngopharyngeal reflux (LPR) as a risk factor in laryngeal cancer.
MethodsWe performed a case-control study with 29 consecutive laryngeal cancer patients who had undergone 24-hour ambulatory double pH monitoring from 2003 to 2006. The control group included 300 patients who had undergone 24-hour ambulatory double pH monitoring due to LPR-related symptoms. We analyzed the prevalence of LPR and numerous parameters from the 24-hour ambulatory double pH monitoring in the laryngeal cancer patient and control groups. Pathologic LPR is defined when more than three episodes of LPR occur in 24 hours.
ResultsThe prevalence of pathologic LPR was significantly higher in the laryngeal cancer group than the control group (P=0.049). The reflux number of the upper probe was significantly higher in the laryngeal cancer group (P<0.001). However the effects of pathologic LPR on laryngeal cancer risk were diluted after adjusting for smoking and alcohol consumption in the multivariable logistic regression.
Laryngeal cancer constitutes 26-30% of all head and neck malignant tumors (1). It is remarkably common in men and it is primarily distributed between ages 40 and 70 years old, but the frequency rate is the highest between ages 50 and 60. Well-known etiologic factors of laryngeal cancer include smoking, alcohol, human papilloma virus and radiation. It has been reported that the occurrence rate is up to 20 times higher in smokers compared to non-smokers, but the exact pathogenesis of the disease is still unknown.
The hypothesis that gastric acid reflux plays a role in the development of squamous cell carcinoma of the larynx was first suggested by Gabriel and Jones (2) in 1960, and it has also been suggested that the chronic stimulation caused by acid reflux leads to a malignant change in the laryngopharyngeal mucosa (3). Since the mid-80's, a high frequency of acid reflux has been reported in laryngeal cancer patients who do not drink alcohol or smoke cigarettes, and this had lead to an increased interest in the relationship between laryngeal cancer and acid reflux (4). Also, it is well known that gastroesophageal reflux (GER) plays a role in the development of cancer of the lower esophagus, suggesting that laryngopharyngeal reflux (LPR) may also play a role in the development of laryngeal cancer (5-7). However, there is still a great deal of controversy surrounding the significance and role of LPR.
Therefore, we performed this study to confirm the significance of LPR as an etiologic factor of laryngeal cancer by measuring the frequency and severity of LPR by 24-hour ambulatory double probe pH monitoring in patients with laryngeal cancer and the control group.
From April 2003 to August 2006, we studied 29 consecutive laryngeal cancer patients who had undergone 24-hour ambulatory double probe pH monitoring. We included all laryngeal cancer patients except those who did not consent to pH monitoring. All of those in the laryngeal cancer group were newly diagnosed and did not receive any previous treatment. Informed consent was obtained from each patient, and the Institutional Review Board of Hanyang University Hospital approved the study protocol.
All laryngeal cancers were squamous cell carcinoma. The average age of the laryngeal cancer group was 62.0┬▒9.45, and there were 27 (93.1%) males and 2 (6.9%) females. The subsites of laryngeal cancer were the glottis in 24 cases (82.8%), and the supraglottis in 5 cases (17.2%).
The control group included 300 patients who had undergone 24-hour ambulatory double-probe pH monitoring due to LPR-related symptoms such as chronic cough, globus sensation, hoarseness and throat clearing during the same period. The control group showed no specific findings of precancerous lesion such as leukoplakia or malignant tumors of the larynx or pharynx. The average age of the control group was 47.7┬▒12.20, and there were 129 (43.0%) males and 171 (57.0%) females.
Twenty four-hour ambulatory double probe pH monitoring was performed to evaluate LPR. Under laryngopharyngeal endoscopy, we inserted a Zinetics 24 double-probe catheter transnasally, and placed the upper probe just above the cricopharyngeus muscle, and the lower probe 15 cm below the upper probe. All findings were recorded for 24 hours using the DigitrapperŌäó pH recorder (Medtronic Inc., Shoreview, MN, USA). Analysis was carried out using the Polygram 98 Diagnostic Workstation (ver. 2.2.0.2558).
A LPR event was defined by an abrupt decrease in pH to below 4 in the upper probe, with an accompanying or preceding decrease in pH to below 4 in the lower probe, except while eating food. Pathologic LPR is defined when more than three episodes of LPR occur (8).
We analyzed whether or not there were differences among the groups in the frequency of pathologic LPR and in parameters measured during the 24-hour ambulatory double-probe pH monitoring, including total episodes of reflux, the percent time in which the pH fell below 4 in the supine, upright, and total position, and the DeMeester Score. The DeMeester score determined by calculating the measured value of the six components: 1) total percent time pH less than 4.0, 2) percent time pH less than 4.0 in the upright position, 3) percent time pH less than 4.0 in the supine position, 4) the total number of reflux episodes, 5) the total number of reflux episodes longer than 5 minutes, and 6) the duration of the longest reflux episode (9). The score is automatically calculated and reported by the Polygram 98 Diagnostic Workstation (ver. 2.2.0.2558) software program.
The SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, the Pearson's chi-square test was used for the comparison of LPR frequency, and a one-way ANOVA method was used for the comparison of the measured parameters during 24-hour ambulatory double-probe pH monitoring. A multivariable logistic regression model was used to adjust age, gender and the well-known risk factors of laryngeal cancer such as tobacco-smoking and alcohol consumption. P<0.05 was considered significant.
The laryngeal cancer group showed a significantly higher frequency of pathologic LPR (86.2%) compared to the control group (70.3%, P=0.049) (Table 1).
Among the 29 cases of laryngeal cancer, there were 15 (51.7%) cases in stage I, 9 (31.0%) cases in stage II, 2 (6.9%) cases in stage III, and 3 (10.4%) cases in stage IV. There was no statistical correlation between the frequency of pathologic LPR and the disease stage.
The average number of total episodes of LPR detected in the laryngeal cancer group was 10.86┬▒8.57, which was significantly higher compared to 5.37┬▒5.42 in the control group (P<0.001). From the upper probe, there were no significant differences noticed between two groups for the percent times for the pH falling below 4 in the supine, upright, and total positions and the DeMeester Scores (Table 2).
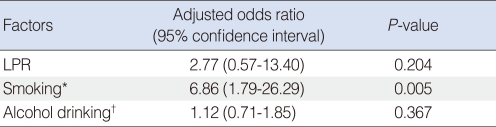
To eliminate the effect of tobacco-smoking and alcohol consumption in the evaluation of LPR and laryngeal cancer risk, we performed multivariable logistic regression test while adjusting for tobacco-smoking and alcohol consumption. The smoker was significantly higher in the laryngeal cancer group (26/29, 89.7%) than the control group (137/300, 45.6%; P<0.001). The alcohol drinker was 17/29 (58.2%) in the laryngeal cancer group and 142/300 (47.3%) in the control group. The alcohol drinker dose not differ between two groups (P=0.224). The adjusted odds ratios and 95% confidence intervals of pathologic LPR, smoking and alcohol drinking were 2.77 (95% confidence interval [CI], 0.57 to 13.40), 6.86 (95% CI, 1.79 to 26.29) and 1.12 (95% CI, 0.71 to 1.85) respectively (Table 3). The analysis showed that only tobacco-smoking is significantly associated with laryngeal cancer risk (P=0.005). The effect of pathologic LPR on laryngeal cancer risk was diluted after the multivariable logistic regression.
LPR is a disease where backflow of gastric contents over the esophageal sphincter produces laryngopharyngeal symptoms such as throat pain, coughing, hoarseness, and globus sensation. It is observed in 10-30% of all patients that visit an otolaryngology clinic, and more than half of the patients have a voice problem or laryngeal disorder that is, in some way, related to LPR. LPR causes numerous chronic laryngeal disorders such as contact granuloma and ulcers, chronic laryngitis, subglottic stenosis, vocal polyps, laryngeal spasms, dysphonia and in the worst case scenario, laryngeal cancer (8, 10, 11).
The mostly highly supported theory for the causal role between LPR and laryngeal cancer is that chronic and repetitive chemical stimulation of gastric acid reflux leads to damage and changes of the laryngeal mucosa (12). This theory is especially supported by research showing that the laryngeal mucosa is much more susceptible to gastric acid than the esophageal mucosa (13). Laryngeal cancer typically develops in the anterior portion of the vocal cords and rarely in the posterior portion of the larynx, where carbonic anhydrase is expressed and neutralizes acid, thereby protecting the posterior portion of the larynx from gastric acid (14). As a result, the anterior portion of the larynx is relatively susceptible to laryngeal cancer because it is not protected from gastric acid. This is indirect evidence that gastric acid causes laryngeal cancer. It is also known that bile, which is included in gastric contents, overexpresses COX-2, which can cause esophageal cancer. It has been suggested that this might also contribute to the development of laryngeal cancer (12). This theory is also supported by an article reporting that achlorhydric patients who have undergone a total gastrectomy have a higher incidence of laryngeal cancer than patients with simple indigestion (15).
There are studies in progress to address whether or not gastric acid reflux is related to laryngeal cancer, but the exact role of gastric acid reflux in the development of laryngeal cancer is still unknown. One study suggested a correlation between GER and laryngeal cancer based on a study of 5 cases (16), and there has been a case report of a non-smoker with a granuloma that developed into laryngeal cancer, which supports the possibility that LPR has a role in the development of laryngeal cancer (17). To evaluate the relationship between LPR and GER with laryngeal cancer, the prevalence rates in laryngeal cancer patients and in a control group need to be compared. However, the difference in prevalence rates between LPR and GER in laryngeal cancer are significant in certain studies but not in others. Some studies reported that GER occurred in 25-67% of laryngeal cancer patients and the incidence was higher in the laryngeal cancer group compared with the control group (5). Copper et al. (18) reported a 62% occurrence rate of LPR and 67% occurrence rate of pathologic GER in laryngeal and pharyngeal cancer patients. Ozlugedik et al. (19) reported a 62% occurrence rate of LPR and a 45% occurrence rate of pathologic GER in laryngeal cancer patients, although there was no significant difference when compared to the control group. In this study, the incidence of pathologic LPR was 86.2% in the laryngeal cancer group, which was much higher than that in the control group (70.3%; P=0.049). Also, the total number of reflux episodes recorded in the upper probe in the laryngeal cancer group was 10.86, which was two times higher than that of the control group (P<0.001). In this study, the frequency of LPR in the patient group and control group were relatively higher compared to that in previous studies in which the sensitivity of machinery and difference in criteria were taken into account.
The criteria for pathologic LPR has not yet been established, although Ozlugedick et al. (19) defined pathologic LPR as having only one episode of reflux in the upper probe and Copper et al. (18) defined pathologic LPR as when the percentage of time that the pH fell below 4 was more than 0.1% of the total time, and/or more than 0.2% of the time in the upright position, and/or more than 0% of the time in the supine position. In this study, we used strict criteria to define pathologic LPR as more than 3 episodes of reflux in 24 hours as previous our study so that we could evaluate its exact role in the development of laryngeal cancer (8).
We were able to confirm that the frequencies and degrees of pathologic LPR are much more severe in the laryngeal cancer group than in the control groups. Based on these results, it could be suggested that pathologic LPR might play a role in the development of laryngeal cancer. However the effects of pathologic LPR on laryngeal cancer risk were diluted after adjusting for smoking and alcohol consumption in the multivariable logistic regression. The analysis showed that only tobacco-smoking is significantly associated with laryngeal cancer risk and the effect of pathologic LPR was diluted. Therefore, it is not clear that pathologic LPR is a real causative factor or cofounder in the development of laryngeal cancer. In order to overcome the limitation of this study, further study with the larger sample size should be necessary while comparing with the true normal control group who has no LPR-related symptoms.
In order to evaluate the exact role of LPR in the development of laryngeal cancer, normal individuals with absolutely no sign of laryngeal symptoms could be selected for the control group. However, executing a 24-hour ambulatory double probe pH monitoring in normal individuals with no laryngeal symptoms is actually very difficult. In a study carried out by Bacciu et al. (6) based on laryngeal cancer patients with no history of smoking or drinking and a normal control group consisting of individuals with no abnormal laryngeal findings, GER was noticed in 27.7% in the laryngeal cancer group and 4.8% in the control group. In our study, the control group consisted of patients with LPR-related symptoms, rather than normal individuals. However, the frequencies of LPR among patients complaining of actual LPR symptoms were higher than those in the normal control group (19). Therefore, we believe that if the occurrence of LPR is exceptionally higher in the laryngeal cancer group than in a control group with LPR-related symptoms the results would be significantly higher when comparing laryngeal cancer patients with a control group of normal individuals.
In conclusion, the prevalence and severity of LPR was significantly higher in laryngeal cancer patients than in the control group with LPR-related symptoms. Therefore, our results support that pathologic LPR might be a possible risk factor in the development of laryngeal cancer although the effect of pathologic LPR on laryngeal cancer risk was diluted after adjusting for smoking and alcohol consumption. A further study with larger sample size should be necessary to verify the exact role of LPR as a risk factor in laryngeal cancer.
References1. Muir C, Weiland L. Upper aerodigestive tract cancers. Cancer. 1995 1 01; 75(1 Suppl):147-153. PMID: 8000993.
2. Gabriel CE, Jones DG. The importance of chronic laryngitis. J Laryngol Otol. 1960 6;74:349-357. PMID: 13825824.
3. Glanz H, Kleinsasser O. Chronic laryngitis and carcinoma. Arch Otorhinolaryngol. 1976 2 08; 212(1):57-75. PMID: 989307.
4. Wight R, Paleri V, Arullendran P. Current theories for the development of nonsmoking and nondrinking laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003 4;11(2):73-77. PMID: 14515082.
5. Geterud A, Bove M, Ruth M. Hypopharyngeal acid exposure: an independent risk factor for laryngeal cancer? Laryngoscope. 2003 12;113(12):2201-2205. PMID: 14660928.
6. Bacciu A, Mercante G, Ingegnoli A, Ferri T, Muzzetto P, Leandro G, et al. Effects of gastroesophageal reflux disease in laryngeal carcinoma. Clin Otolaryngol Allied Sci. 2004 10;29(5):545-548. PMID: 15373871.
7. El-Serag HB, Hepworth EJ, Lee P, Sonnenberg A. Gastroesophageal reflux disease is a risk factor for laryngeal and pharyngeal cancer. Am J Gastroenterol. 2001 7;96(7):2013-2018. PMID: 11467626.
8. Chung JH, Tae K, Lee YS, Jeong JH, Cho SH, Kim KR, et al. The significance of laryngopharyngeal reflux in benign vocal mucosal lesions. Otolaryngol Head Neck Surg. 2009 9;141(3):369-373. PMID: 19716016.
9. Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus: a quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974 10;62(4):325-332. PMID: 4432845.
10. Shin KS, Tae K, Jeong JH, Jeong SW, Kim KR, Park CW, et al. The role of psychological distress in laryngopharyngeal reflux patients: a prospective questionnaire study. Clin Otolaryngol. 2010 2;35(1):25-30. PMID: 20447159.
11. Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002 7;127(1):32-35. PMID: 12161727.
12. Wilson JA. What is the evidence that gastroesophageal reflux is involved in the etiology of laryngeal cancer? Curr Opin Otolaryngol Head Neck Surg. 2005 4;13(2):97-100. PMID: 15761283.
13. Garrigues V, Gisbert L, Bastida G, Ortiz V, Bau I, Nos P, et al. Manifestations of gastroesophageal reflux and response to omeprazole therapy in patients with chronic posterior laryngitis: an evaluation based on clinical practice. Dig Dis Sci. 2003 11;48(11):2117-2123. PMID: 14705815.
14. Maronian N, Haggitt R, Oelschlager BK, Bronner M, Yang J, Reyes V, et al. Histologic features of reflux-attributed laryngeal lesions. Am J Med. 2003 8 18; 115(Suppl 3A):105S-108S. PMID: 12928084.
15. Galli J, Cammarota G, Calo L, Agostino S, D'Ugo D, Cianci R, et al. The role of acid and alkaline reflux in laryngeal squamous cell carcinoma. Laryngoscope. 2002 10;112(10):1861-1865. PMID: 12368631.
16. Olson NR. Effects of stomach acid on the larynx. Proc Am Laryngol Assoc. 1983;104:108-112.
17. Ward PH, Hanson DG. Reflux as an etiological factor of carcinoma of the laryngopharynx. Laryngoscope. 1988 11;98(11):1195-1199. PMID: 3185074.
18. Copper MP, Smit CF, Stanojcic LD, Devriese PP, Schouwenburg PF, Mathus-Vliegen LM. High incidence of laryngopharyngeal reflux in patients with head and neck cancer. Laryngoscope. 2000 6;110(6):1007-1011. PMID: 10852522.
19. Ozlugedik S, Yorulmaz I, Gokcan K. Is laryngopharyngeal reflux an important risk factor in the development of laryngeal carcinoma? Eur Arch Otorhinolaryngol. 2006 4;263(4):339-343. PMID: 16252124.
|
|
||||||||||||||||||||||||||||||||||||||