|
 |
- Search
AbstractObjectivesCefditoren pivoxil (CDT) has been used in the treatment of rhinosinusitis. However, little is known about the efficacy of this drug at low and high doses. This study was to compare the efficacy and safety of low dose (8-12 mg/kg/day) and high dose (16-20 mg/kg/day) CDT in the treatment of children with uncomplicated acute rhinosinusitis (ARS).
MethodsThis investigation was a randomized, investigator-blinded, and parallel study, conducted in patients (aged 1-15 years) with a clinical diagnosis of uncomplicated ARS. Two groups of patients randomly received low dose or high dose CDT for 14 days. Patients' symptoms were assessed quantitatively using a quantitative symptom score (the S5 score). The changes in sinus symptoms and adverse events were provided by patients and their parents/caregivers. The response rate and adverse effects were evaluated at days 7 and 14. The relapse rate was recorded at days 21 and 28. The recurrences of sinus symptoms at day 60 were also assessed.
ResultsOne hundred forty patients were recruited and randomized; 72 received low dose CDT (group I) and 68 received high dose CDT (group II). There were no significant differences in demographic data including sex, age, presenting symptoms, medical history, and X-ray findings between two groups. The responses rate at day 14 in groups I and II were 95.5% and 95.4%, respectively (P>0.99). There were no significant differences between groups in relapse rate at day 28 and no recurrence at day 60 in either group. The most common treatment-related adverse events were diarrhea (4.2% in group I vs. 2.9% in group II) and vomiting (2.8% in group I vs. 10.3% in group II). There was no statistically significant difference in adverse events between groups.
Acute rhinosinusitis is a common childhood disease. It usually occurs following a viral upper respiratory tract infection (URI) and may also occur with allergies that causes inflammation of the mucous membranes. Most cases begin as a common cold. Symptoms often go away within a week to 10 days but in some patients, a bacterial infection, most commonly caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis could develop. The increasing prevalence of antimicrobial resistance in many areas has been a therapeutic challenge [1]. The prevalence of penicillin-nonsusceptible S. pneumoniae increased from 10.4%-14.1% in 1996-1997 to 22.3% in 1999-2000 [2,3]. In 2009-2010, the CARTIPS (community-acquired respiratory tract infection pathogen surveillance) program, a study in Asia that used breakpoints for oral penicillin V recommended by the Clinical and Laboratory Standards Institute, revealed the prevalence of penicillin-non-susceptible S. pneumoniae to be between 46% and 100%. Azithromycin and clarithromycin had variable resistance rates of 0%-88% amongst S. pneumoniae. β-Lactamase production rates ranged from 15% to 46.6% amongst H. influenzae isolates and from 90% to 100% amongst M. catarrhalis isolates [4]. Data from the National Antimicrobial Resistance Surveillance Thailand, during 2000 to 2005 showed that the rates of penicillin resistance were constantly high, ranging from 42.4% in 2000 to 47.7% in 2005. The third-generation cephalosporin resistance rate, determined by Epsilon test in 10% to 15% of all isolates each year, ranged from 2.1% to 8.4%. The rates of erythromycin resistance ranged from 24.2% to 30.3% [5]. Because of these changes in epidemiology, new treatment strategies for many infectious diseases including acute bacterial rhinosinusitis are crucial.
Once acute bacterial rhinosinusitis is diagnosed, treatment with antibiotic is indicated. Most treatment is done empirically based on information about which bacteria are most likely to be causing the infection. Antimicrobial regimens for children with acute bacterial rhinosinusitis include high doses of amoxicillin, amoxicillin/clavulanate, cefpodoxime proxetil, cefuroxime axetil, or cefdinir. Trimethoprim/sulfamethasoxazole, azithromycin, clarithromycin, or erythromycin are recommended if the patient has a history of immediate type I hypersensitivity reaction to β-lactams [6]. Cefditoren pivoxil (CDT; Meiact, Meiji Seika Pharma Co., Tokyo, Japan) is a third-generation oral cephalosporin with good activity against respiratory tract pathogens, including penicillin-intermediate and penicillin-resistant strains of S. pneumoniae as well as beta-lactamase producing strains of H. influenzae and M. catarrhalis [7,8,9,10,11]. For CDT, as for β-lactams, the time (t) (expressed as percentage of the dosing interval) that antibiotic concentrations exceed the value of minimum inhibitory concentration (MIC) (t>MIC) is the index predicting efficacy with a cutoff value of 40% for clinical cure in humans [12,13]. When given at 3 mg/kg three times a day (9 mg/kg/day) of cefditoren features 1.45 mcg/mL of Cmax and a 2.25-hour half-life and 6 mg/kg (three times a day; 18 mg/kg/day) features 2.85 mcg/mL of Cmax and a 1.68-hour half-life [14], indicates that adequate pharmacodynamic indexes covering all H. influenzae, and most S. pneumoniae isolates can be achieved. This makes cefditoren an antibiotic that may play a significant role in the treatment of bacterial respiratory tract infections in the community [15]. In the clinical setting, studies carried out with cefditoren showed that treatments with the 400 mg twice a day regimen were associated with high rates of bacteriological response, even against penicillin nonsusceptible S. pneumoniae, with good correlation between bacteriological efficacy/response and clinical outcome [15], consistent with the pharmacokinetic profile of cefditoren [16]. However, little is known about the efficacy of this drug at low and high doses. The objective of the present study was to compare the efficacy and safety of low dose (8-12 mg/kg/day) and high dose (16-20 mg/kg/day) CDT in the treatment of children with acute rhinosinusitis.
This investigation was a randomized, investigator-blinded, and parallel study, conducted in patients (age 1-15 years) with a clinical diagnosis of uncomplicated acute rhinosinusitis: persistent URI symptoms for >10 days, high fever (>39℃) with purulent nasal discharge at least 3-4 consecutive days and the new onset of fever, or increase in nasal discharge following a typical viral URI that lasted 5-6 days and were initially improving [6]. For confirmation of the diagnosis, radiography of the paranasal sinus was performed and interpreted by a blinded radiologist. Eligible patients were recruited from the Pediatric Allergy Clinic and Pediatric Outpatient Clinic of Thammasat University Hospital, Pathumthani, Thailand. The inclusion criteria were age >1 year, diagnosed rhinosinusitis, pretreatment of antibiotics within 3 months or age 1-2 years and positive radiographic findings (opacification of paranasal sinuses, >33% loss of air-space volume within the maxillary sinuses, or >4 mm of mucosal thickening in the maxillary sinuses). Exclusion criteria included chronic sinus symptoms for >4 weeks, immunocompromised status, hypersensitivity to penicillins or cephalosporins.
Two groups of patients randomly received low dose (4-6 mg/kg) or high dose CDT (8-10 mg/kg), given as sachets or tablets twice a day (maximum 400 mg/day), for 14 days. A computer-generated randomization schedule was used to assign patients in a 1:1 ratio to treatment groups by using a coded list. Investigators were blinded to treatment assignments. Study medication was dispensed by a research assistant not involved in the study assessments. Use of antibiotics other than the study drugs was prohibited throughout the study. There were no restrictions on the use of medications for symptomatic relief (e.g., decongestants, antihistamines, intranasal corticosteroids). The protocol was approved by the Institutional Review Board of the Faculty of Medicine, Thammasat University. All parents or legal representatives of the screened and enrolled patients provided written informed consent before initiation of study treatment. The study was performed in accordance with the Declaration of ClinicalTrial.gov (ClinicalTrials.gov Identifier: NCT01553006).
The baseline evaluation including a medical history and physical examination (including nasal inspection) was conducted. Patients' symptoms were assessed quantitatively using the S5 questionnaire [17], a validated and reliable instrument developed specifically for the evaluation of sinus symptoms in children. The S5 score is the mean of symptom scores for nasal obstruction, daytime and nighttime coughing, headache, and colored nasal discharge (0, absent; 1, small problem; 2, moderate problem; 3, large problem). The S5 score was calculated by the physician at the initial office visit and by the parent/caregiver at home each day throughout the course of treatment. A research assistant performed randomization for each patient using the computer program as described. A research assistant trained caregivers in recording this questionnaire and confirmed that they could do so correctly. The clinical status of patients was assessed by the investigators between treatment days 7 and 14, and after treatment (days 21 and 28). On day 7 and 14 after the initial visit, patients returned for an assessment of clinical signs and symptoms of acute bacterial rhinosinusitis (ABRS), monitoring of adverse events and compliance. The same research assistant conducted a telephone follow-up every day during treatment for checking compliance and side effects. In addition, the compliance was checked by counting the number of sachets/tablets remaining in each medication container at each visit. Information on adverse events, concomitant medication use, and compliance was collected using a scripted questionnaire. Investigators assessed the severity and causality of each adverse event and its relationship to study drug. Changes in study drug administration and withdrawals from the study were also recorded. At the end-of-therapy visit on day 14, rates of improvement were assessed by investigators. The relapse rate was recorded by investigators at days 21 and 28. The recurrence of sinus symptoms was indirectly assessed at the day-60 telephone follow-up by questioning the caregiver about clinical symptoms. The investigator assessed the clinical response and side effects while still blinded to the study drug assignment. All assessments made by the same investigator for each subject.
The primary efficacy end point was clinical response, as determined by the investigator at the end-of-therapy visit on day 14. The clinical response was classified as improvement or failure. Improvement was defined as the resolution of >1 symptoms of ABRS and no requirement for additional antibiotics for the treatment of rhinosinusitis. Failure was defined as lack of improvement in the acute signs or symptoms of ABRS or the worsening of >1 sign or symptom of the original infection after >3 days of the study drug. The secondary efficacy end points included rates of relapse and recurrence. Relapse was defined as a subjective rating of lack of improvement at day 21 or 28 in a patient rated as improved on day 14. Recurrence was defined as sinus symptoms remaining for >10 days during the second month of follow-up in a patient rated as improved on day 28.
Sample-size calculations were based on a chi-square test with a power of ≥80% and were performed using the power calculators by SealedEnvelope (Sealed Envelope, London, UK). Assuming response rates of 65% for CDT I and 40% for CDT II, it was determined that it would be necessary to enroll 79 patients in each group.
The primary efficacy end point was analyzed in the clinically evaluable population, which included all patients who met the study inclusion criteria and end-of-therapy assessment and received no other antibiotics before the end-of-therapy visit.
Because of the nonnormality of the data, medians and interquartile ranges were used for continuous variables. Categorical variables were expressed as percentages. Demographic and clinical characteristics of the study groups were compared using the Fisher exact test (2 tailed), the Mann-Whitney U-test, or the Cramer V coefficient, as appropriate. The Fisher exact test was used to compare radiographic findings and the use of concomitant medication between groups. Differences in treatment durations and outcomes between groups were analyzed using the Mann-Whitney U-test. A comparison of adverse effects between groups was conducted using the Fisher exact test. Values were considered statistically significant at P<0.05. All analyses were performed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
One hundred forty patients were recruited and received follow-ups. A group of 72 patients randomized to receive low dose CDT (group I) composed of 37 males and 35 females with a median age of 4 years. A group of 68 patients randomized to receive high dose of CDT (group II) composed of 34 males and 34 females with median age of 5 years. There were no significant differences between groups in terms of sex, age, presenting symptoms, medical history, home environment, or day care attendance. At baseline, the majority of patients had rhinorrhea, nasal obstruction and cough. A large proportion of patients' families had >1 children at home (64.7% and 64.7%), most with <2 episodes in the previous year (85.9% and 89.7%). Most patients had received antibiotics in the previous 90 days (68.1% and 66.2%). There were no significant differences between groups in terms of the antibiotics used in the 90 days before the study. Detailed demographic and clinical characteristic data for the two groups at enrollment are shown in Table 1.
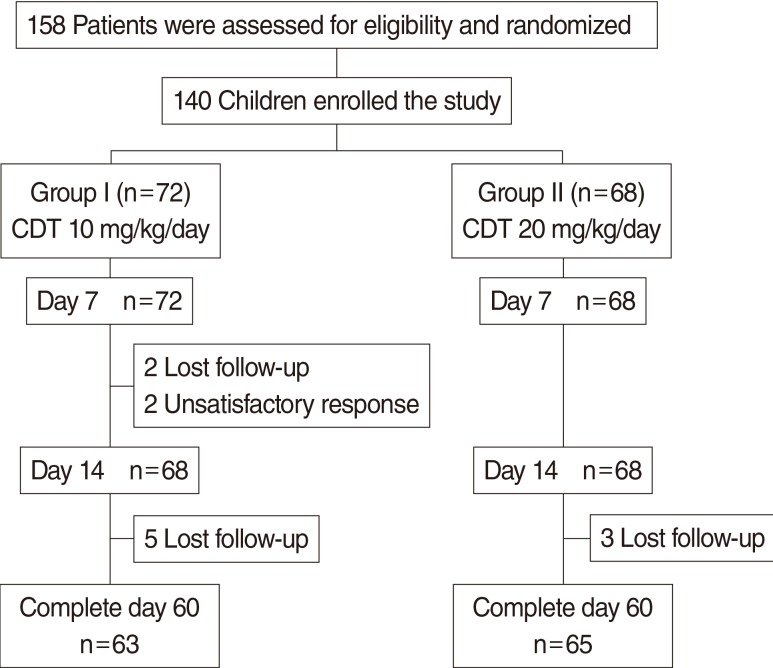
The flow of the study is illustrated in Fig. 1. Most patients completed medication; 94.4% in group I and 100% in group II. Four patients in group I were lost to follow up within 2 weeks and we were not able to track them even with multiple phone calls or letters. The reasons for withdrawal from the study group were patient dissatisfaction with treatment.
Table 2 shows the radiographic findings in the two study groups. Most patients in both groups had maxillary involvement (94%). The most common abnormal finding from sinus radiography was mucosal thickening. Diffuse opacification was observed in 47.1% and 56.1% of patients. There were no statistically significant differences between groups in radiographic findings. The concomitant medications used are shown in Table 3. There was no significant difference in the use of concomitant treatments during the study.
The change in S5 scores in the two groups at day 3, 7, and 14 were not significantly different. Favorable responses at day 14 in groups I and II were 95.5% and 95.4%, respectively (P>0.99). At day 28, the relapse rate in group I was higher than in group II (15.5% vs. 4.8%). There was no recurrence at day 60 in either group. The differences in rate of relapse and recurrence were not statistically significant between groups (Table 4).
The most common treatment-related adverse events were diarrhea (4.2% in group I vs. 2.9% in group II) and vomiting (2.8% in group I vs. 10.3% in group II) (Table 5). The reported diarrhea and vomiting were mild symptoms that resolved spontaneously without discontinuation of the antibiotic. There was no statistically significant difference in adverse events between groups. No serious adverse events were observed during the study, and no patient discontinued the study because of adverse events.
Rhinosinusitis is one of the most common pediatric infectious diseases and antimicrobials are most frequently used in treatment. Common causative organisms include S. pneumoniae, M. catarrhalis, and H. influenzae. Thailand has just had the vaccine available in 2006. It was not included in the National Vaccine Program with low vaccine uptake rate among children under 5 years of age [18]. Thus, the incidence of diseases caused by this organism would not be expected to change while the incidence of resistance might have been evolved over time due to changing pattern of antibiotic prescription.
Cefditoren has a broad spectrum of activity against gram-positive and gram-negative bacteria, including common sinus pathogens. Cefditoren has shown excellent in vitro activity against the penicillin-susceptible and penicillin-intermediately susceptible S. pneumoniae, β-lactamase-positive and β-lactamase-negative H. influenzae, and M. catarrhalis [19]. Thus, this agent could be a viable alternative for the treatment of infections caused by these organisms. High dose treatment might reduce the chance of emergence of resistance during therapy as has been proposed with another agent [20]. We tried to determine whether higher dose would give rise to a better outcome as well as to assess the side effects of using high dose therapy. We found similar rate of clinical response and adverse events between low- and high-dose regimens.
Clinical efficacy of cefditoren was demonstrated in a previous study involving treatment of bacterial rhinosinusitis in children comparing usual dose (4-6 mg/kg twice a day) CDT versus 80-90 mg/kg (on amoxicillin basis) amoxicillin/clavulanic acid (maximum dose 800 mg/day) twice a day. These two treatment arms showed similar clinical improvement, relapse rates or recurrences of sinus symptoms in both groups [21]. Given at the dose of 3 and 6 mg/kg, peak serum concentrations of 1.54 and 2.85 µg/mL were obtained, respectively. This suggests that cefditoren has linear (or dose-dependent) pharmacokinetics and higher dose might be associated with a better outcome. The double-blind phase I study conducted to evaluate the pharmacokinetics and the pharmacodynamics of cefditoren following single-dose and multiple twice a day and three times a day regimens in healthy volunteers showed the mean T>MIC was always above 40% for both the twice a day and three times a day regimens. There were no differences between single-dose and multiple twice a day and three times a day regimens groups in the incidence of gastrointestinal adverse events [16]. A CDT 400-mg twice a day regimen in healthy volunteers obtains the following T>MIC rates: 55% for MIC of 0.5 µg/mL, 68% for MIC of 0.25 µg/mL, 81% for MIC of 0.12 µg/mL and 94% for MIC of 0.06 µg/mL [16]. CDT has shown a postantibiotic effect greater than 1 hour for S. pneumoniae, which also supports the twice daily regimen for the treatment of respiratory tract infections [22].
In this study, we obtained similar response rates between low and high dose cefditoren for treatment of uncomplicated ARS in pediatric patients. The difference in rates of relapse and recurrence were not statistically significant between the two groups. However, the relapse rate in the low dose group was higher than in the high dose group (15.5% vs. 4.8%).
A previous study comparing oral CDT 200 or 400 mg twice a day with either cefuroxime 250 mg twice a day or cefadroxil 500 mg twice a day for the treatment of uncomplicated skin and skin-structure infections showed that the clinical cure rate and tolerability of CDT 200 or 400 mg twice a day were comparable to those of cefuroxime and cefadroxil [23]. In the pooled analysis of the three studies in adults with acute sinusitis, there were no differences found in clinical response between CDT 200 mg twice a day or 400 mg twice a day and comparators (cefuroxime or amoxicillin/clavulanic acid) at the end of therapy (80.2% vs. 84.8%) and at the end of follow-up (71.2% vs. 77.4%) [24]. However, a study in community pneumonia and acute exacerbations of chronic bronchitis showed that treatments with the CDT 400 mg twice a day regimen were associated with high rates of bacteriological response, even against penicillin nonsusceptible S. pneumoniae, with good correlation between bacteriological efficacy/response and clinical outcome [25]. An increased dose of CDT (6 mg/kg three times a day) in patients with acute otitis media showed the efficacy rate of 89% for Penicillin-resistant S. pneumoniae, 86% for Penicillin Intermediate S. pneumoniae and 100% for beta-lactamase negative ampicillin-resistant H. influenzae (BLNAR). The eradication of penicillin-resistant S. pneumoniae can be achieved in about half of patients by administration of CDT at a double dose for 2 weeks. The eradication of BLNAR strains is achieved in 70% of patients by the same treatment. The recommended regimen of CDT is a low or a high dose regimen depending on the indication.
In our study, there was no significant difference in the incidence of side effects between low and high dose CDT, which is in accordance with a previous study [16]. Safety data from 13 clinical trials of CDT on community acquired respiratory infections showed an adverse event profile of CDT similar to those of standard antibiotics used in the treatment of respiratory tract infections. Overall diarrhea related to CDT administration was 9%, while dyspepsia and abdominal pain were reported as adverse events in <2.7% patients [26].
Our study did not reveal a difference between low and high dose regimens of CDT in terms of clinical efficacy for the vast majority of patients who have uncomplicated ARS. The relapse rate in the low dose group was higher than in the high dose group, but this was not statistically significant different. In addition, there was no significant difference in side effects between the two groups. We did not have in-depth details of infecting organisms as well as the minimal inhibitory concentrations of the drug against these causative organisms in the two treatment groups because our patients were treated empirically to simulate routine clinical practice. Since baseline characteristics and the severity of ABRS were similar, it is possible that higher number of patients in the low dose group had relapsed, although not statistically significant, might be due to differences in microbiological profile. Therefore, the role of high dose CDT for the treatment of ABRS in areas with a greater incidence of resistance and higher MIC needs to be further delineated, particularly in terms of microbiological cure rates and emergence of resistance during treatment.
There are several limitations to our study. First, inclusion criteria may overlap patients with viral upper respiratory illness. Viral upper respiratory illness and acute rhinosinusitis are part of a continuum of diseases with an overlapping clinical picture. Inclusion of many patients with viral upper respiratory illness could affect the validity of our findings. However, we randomly enrolled patients with fulfill criteria for the diagnosis of bacterial rhinosinusitis suggested by Infectious Diseases Society of America guideline [6]. Second, we do not have the microbiological data, including MIC of causative bacteria, to correlate between the clinical response and patient microbiological response, as confirmation of a bacterial etiology of rhinosinusitis is not done in routine clinical practice since it requires an antral puncture or, at least, an endoscopic sampling of the middle meatus. Third, the administration of adjunctive symptom-relief medications could potentially blunt differences between compared treatments in trials of ARS.
In conclusion, our data suggested that 10 mg/kg/day and 20 mg/kg/day twice a day regimens of CDT may provide a similar clinical outcome for treatment in uncomplicated ARS in pediatric patient. The administration of CDT 20 mg/kg/day twice a day regimen is well tolerated. The role of high dose cefditoren in the treatment of complicated ARS should be further explored, particularly in the areas where high level resistance among sinus pathogens is prevalent.
ACKNOWLEDGMENTSWe would like to thank our patients and their parents for their kind cooperation. This study was supported by the Faculty of Medicine, Thammasat University.
CONFLICT OF INTERESTCONFLICT OF INTEREST: This study was supported by grant from Thai Meiji Pharmaceutical Industry. The design, conduct, and analysis of data were performed independent of the company's involvement. The company also had no role in the writing or approving the content of this manuscript. All authors have indicated that they have no conflicts of interest regarding the content of the manuscript. References1. Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011 4;37(4):291-295. PMID: 21382699.
2. Felmingham D, Gruneberg RN. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000 2;45(2):191-203. PMID: 10660501.
3. Felmingham D. Evolving resistance patterns in community-acquired respiratory tract pathogens: first results from the PROTEKT global surveillance study. Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin. J Infect. 2002 2;44(Suppl A):3-10. PMID: 12150493.
4. Wang H, Chen M, Xu Y, Sun H, Yang Q, Hu Y, et al. Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009-2010. Int J Antimicrob Agents. 2011 11;38(5):376-383. PMID: 21880469.
5. Dejsirilert S, Tienkrim S, Ubonyaem N, Sawanpanyalert P, Aswapokee N, Suankratay C. National antimicrobial resistance surveillance among clinical isolates of Streptococcus pneumoniae in Thailand. J Med Assoc Thai. 2009 8;92(Suppl 4):S19-S33. PMID: 21298844.
6. Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012 4;54(8):e72-e112. PMID: 22438350.
7. Wellington K, Curran MP. Cefditoren pivoxil: a review of its use in the treatment of bacterial infections. Drugs. 2004;64(22):2597-2618. PMID: 15516158.
8. Soriano F, Granizo JJ, Fenoll A, Gracia M, Fernandez-Roblas R, Esteban J, et al. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae isolated in four southern European countries (ARISE project) from adult patients: results from the cefditoren surveillance program. J Chemother. 2003 4;15(2):107-112. PMID: 12797384.
9. Seral C, Suarez L, Rubio-Calvo C, Gomez-Lus R, Gimeno M, Coronel P, et al. In vitro activity of cefditoren and other antimicrobial agents against 288 Streptococcus pneumoniae and 220 Haemophilus influenzae clinical strains isolated in Zaragoza, Spain. Diagn Microbiol Infect Dis. 2008 10;62(2):210-215. PMID: 18715733.
10. Gracia M, Diaz C, Coronel P, Gimeno M, Garcia-Rodas R, del Prado G, et al. Antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis isolates in eight Central, East and Baltic European countries in 2005-06: results of the Cefditoren Surveillance Study. J Antimicrob Chemother. 2008 5;61(5):1180-1181. PMID: 18316820.
11. Kang JH, Lee SY, Kim JH, Hur JK, Lee KY. In vitro antimicrobial activity of cefditoren and other oral antibiotics against Streptococcus pneumoniae, isolated from children with community acquired respiratory tract infections. Jpn J Antibiot. 2010 2;63(1):11-17. PMID: 20836403.
12. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998 1;26(1):1-10. PMID: 9455502.
13. Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR, Musher DM, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med. 2000 5;160(10):1399-1408. PMID: 10826451.
14. Fujii R, Yoshioka H, Okuno A, Fujita K, Murono K, Maruyama S, et al. Pharmacokinetic and clinical studies of cefditoren pivoxil in the pediatric field: Pediatric Study Group of ME1207. Jpn J Antibiot. 1993 1;46(1):95-114. PMID: 8455336.
15. Soriano F, Gimenez MJ, Aguilar L. Cefditoren in upper and lower community-acquired respiratory tract infections. Drug Des Devel Ther. 2011 2;5:85-94.
16. Sadaba B, Azanza JR, Quetglas EG, Campanero MA, Honorato J, Coronel P, et al. Pharmacokinetic/pharmacodynamic serum and urine profile of cefditoren following single-dose and multiple twice- and thrice-daily regimens in healthy volunteers: a phase I study. Rev Esp Quimioter. 2007 3;20(1):51-60. PMID: 17530036.
17. Garbutt JM, Gellman EF, Littenberg B. The development and validation of an instrument to assess acute sinus disease in children. Qual Life Res. 1999 5;8(3):225-233. PMID: 10472153.
18. Wongsawat J, Chokephaibulkit K. Implication of pneumococcal conjugate vaccines to public health: Thailand perspective. J Med Assoc Thai. 2010 11;93(Suppl 5):S53-S60. PMID: 21294383.
19. Fenoll A, Aguilar L, Robledo O, Gimenez MJ, Tarrago D, Granizo JJ, et al. Influence of the beta-lactam resistance phenotype on the cefuroxime versus cefditoren susceptibility of Streptococcus pneumoniae and Haemophilus influenzae recovered from children with acute otitis media. J Antimicrob Chemother. 2007 8;60(2):323-327. PMID: 17562681.
20. Schrag SJ, Pena C, Fernandez J, Sanchez J, Gomez V, Perez E, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001 7;286(1):49-56. PMID: 11434826.
21. Poachanukoon O, Kitcharoensakkul M. Efficacy of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of pediatric patients with acute bacterial rhinosinusitis in Thailand: a randomized, investigator-blinded, controlled trial. Clin Ther. 2008 10;30(10):1870-1879. PMID: 19014842.
22. Felmingham D, Robbins MJ, Ghosh G, Bhogal H, Mehta MD, Leakey A, et al. An in vitro characterization of cefditoren, a new oral cephalosporin. Drugs Exp Clin Res. 1994;20(4):127-147. PMID: 7813385.
23. Bucko AD, Hunt BJ, Kidd SL, Hom R. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clin Ther. 2002 7;24(7):1134-1147. PMID: 12182257.
24. Granizo JJ, Gimenez MJ, Barberan J, Coronel P, Gimeno M, Aguilar L. Efficacy of cefditoren in the treatment of upper respiratory tract infections: a pooled analysis of six clinical trials. Rev Esp Quimioter. 2008 3;21(1):14-21. PMID: 18443928.
25. Granizo JJ, Gimenez MJ, Barberan J, Coronel P, Gimeno M, Aguilar L. The efficacy of cefditoren pivoxil in the treatment of lower respiratory tract infections, with a focus on the per-pathogen bacteriologic response in infections caused by Streptococcus pneumoniae and Haemophilus influenzae: a pooled analysis of seven clinical trials. Clin Ther. 2006 12;28(12):2061-2069. PMID: 17296462.
26. Granizo JJ, Aguilar L, Gimenez MJ, Coronel P, Gimeno M, Prieto J. Safety profile of cefditoren: a pooled analysis of data from clinical trials in community-acquired respiratory tract infections. Rev Esp Quimioter. 2009 6;22(2):57-61. PMID: 19544097.
Table 1.Demographic and clinical characteristics of pediatric treated with cefditoren pivoxil (low dose) and cefditoren pivoxil (high dose)
Table 2.Radiographic findings in both groups
Table 3.Concomitant treatments used during the study by patients in the cefditoren pivoxil (low dose) and cefditoren pivoxil (high dose) treatments groups
Table 4.Treatment outcomes in patients in the cefditoren pivoxil (low dose) and cefditoren pivoxil (high dose) treatments groups
Table 5.Adverse events occurring in patients in the cefditoren pivoxil (low dose) and cefditoren pivoxil (high dose) treatment groups
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||