Clinical and Laboratory Features of Various Criteria of Eosinophilic Chronic Rhinosinusitis: A Systematic Review and Meta-Analysis
Article information
Abstract
Objectives
The aim of this study was to evaluate the differences in clinical and laboratory features between eosinophilic chronic rhinosinusitis (ECRS) and non-ECRS and to compare diagnostic criteria for ECRS.
Methods
We compared clinical features and/or laboratory findings classified as ECRS and non-ECRS according to various diagnostic criteria (histological and clinical). We also analyzed studies to compare endoscopic findings, symptom scores, laboratory findings, and computed tomography (CT) findings between ECRS and non-ECRS.
Results
Our search included 55 studies with 6,143 patients. A comparison of clinical features and/or laboratory criteria with histological criteria showed no significant differences in nasal symptom scores and CT scores according to criteria. Serum eosinophil levels showed differences across the criteria, with ECRS consistently characterized by higher serum eosinophil levels than non-ECRS. Among the four criteria, the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) criteria and tissue eosinophilia (≥70) were associated with decreased olfactory function. In laboratory findings, the eosinophil percentage (standardized mean difference [SMD], 1.561; 95% confidence interval [CI], 1.329–1.794; P<0.001) and eosinophil count (SMD, 1.493; 95% CI, 1.134–1.852; P<0.001) of eosinophils were higher in ECRS than non-ECRS. In clinical findings, nasal symptom scores (SMD, 0.382; 95% CI, 0.156–0.608; P<0.001), endoscopic nasal polyp scores (SMD, 0.581; 95% CI, 0.314–0.848; P<0.001), and olfactory dysfunction (SMD, 0.416; 95% CI, 0.037–0.794; P=0.031) were higher in ECRS than in non-ECRS. With regard to CT findings, the whole-sinus opacification score (SMD, 0.824; 95% CI, 0.588–1.059; P<0.001) was higher in ECRS than in non-ECRS. In particular, there were significant differences in anterior ethmoid sinus and sphenoid sinus opacification.
Conclusion
ECRS and non-ECRS differ in their clinical and laboratory features. When histological confirmation is difficult on an outpatient basis, ECRS could be diagnosed using clinical features and/or laboratory findings.
INTRODUCTION
Chronic rhinosinusitis (CRS) is a common chronic otolaryngological disease, the classification and treatment of which are still being discussed. Patients with CRS have generally been classified according to their clinical phenotype (i.e., with or without polyps). In recent years, CRS patients have been classified according to endotype, which characterizes the pathogenesis of the disease according to the inflammatory process [1]. Based on the presence or absence of tissue eosinophilic infiltration, CRS can also be divided into eosinophilic CRS (ECRS) and nonECRS subtypes [2]. Eosinophilic and non-eosinophilic airway inflammation can present with several different clinical symptoms [3]. Non-ECRS can be controlled relatively well with a combination of endoscopic sinus surgery and low-dose macrolide therapy. By contrast, in patients with ERCS, nasal polyps tend to recur frequently after endoscopic sinus surgery [4]. In addition, ECRS does not respond well to macrolide therapy, but shows a good initial response to systemic steroid therapy in recurrent cases [4].
A number of studies have compared the differences between ECRS and non-ECRS with nasal polyps, but no consensus yet exists regarding the histopathological criteria for diagnosis [4]. Some studies have defined ECRS using various tissue eosinophilia cutoff values, such as 5, 8, 10, 70, 100, or 120 eosinophils per high-power field (HPF; ×400), whereas others have defined it using the proportion of eosinophils to the total number of inflammatory cells based on various cutoff values such as 5%, 10%, 11%, 20%, or up to 50%. Inconsistencies also exist regarding the measurement method. The Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC) proposed criteria for defining ECRS that were based not on tissue eosinophilia, but on a scoring system composed of clinical findings, such as disease site (bilateral sinus involvement), the presence of nasal polyps, computed tomography (CT) findings (soft tissue density in ethmoid sinuses compared to the maxillary sinuses), and serum eosinophilia [5]. As the treatment strategy differs for ECRS and non-ECRS, it would be useful to have an effective standard for diagnosing ECRS in outpatient clinics without the need to collect and analyze sinus tissue or nasal polyps [4]. Therefore, we analyzed the clinical and laboratory features of ECRS and non-ECRS and compared the criteria that can be used in the clinic without nasal biopsy with histopathological criteria.
MATERIALS AND METHODS
Study protocol and registration
This systematic review and meta-analysis is described with reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [6]. This study protocol was registered prospectively on Open Science Framework (https://osf.io/enb3j).
Literature search
Clinical studies were identified in PubMed, Scopus, Embase, the Web of Science, and the Cochrane Central Register of Controlled Trials up to November 2021. The search terms were as follows: “sinusitis,” “nasal polyps,” “eosinophils,” “cell count,” “eosinophilic chronic sinusitis,” “blood eosinophil percentage,” “olfactory,” “nasal endoscopy score,” “computed tomography score,” “symptom,” and “visual analog scale.” Two reviewers (DHK and MAB) independently reviewed the titles and abstracts of candidate studies in each database and excluded irrelevant studies. If the two reviewers did not agree on a paper, its eligibility for inclusion was determined through discussion with a third reviewer (SWK). Papers that lacked quantifiable or relevant data were excluded after a full-text review. References of included studies were also searched to ensure that no related studies were omitted.
Selection criteria
Inclusion criteria were as follows: patients underwent clinical or imaging studies on ECRS, prospective or retrospective study, comparison of clinical or imaging data with non-ECRS data, and data on results of mean differences or odds ratio (OR) analyses. Exclusion criteria were as follows: case report; review article; report of other rhinological disease, such as rhinitis or septal deviation; non-English language article; and lack of laboratory, clinical, or imaging data for statistical analysis. The search and selection strategy is summarized in Fig. 1.
Data organization and risk of bias assessment
Data were extracted from selected eligible studies and organized in a standardized format [7,8]. We abstracted data on numbers of patients, sex, nationality, diagnostic criteria, outcomes of additional tests performed to evaluate ECRS, and the P-values for comparisons of ECRS and non-ECRS. Analyzed outcomes were the percentage or absolute count of eosinophils [3-5,9-51], total non-specific immunoglobulin E (IgE) [3,4,13,15,16,19,21-23,30,35,39-41,48,52-54], endoscopic polyp score [3,9,28-30,32,33,37,38,40,41,43,51,52,54-59], nasal symptomatic score [3,31,33,37,40,41,46,50-52,55-58,60], olfactory function [23,25-27,30,37,41,42,48,56], CT score [3,4,9-14,16,23-26,28-33,35,37,38,40,41,43-45,47,48,50-53,55-61], and the odd ratios of comorbidities associated with ECRS (allergic rhinitis, asthma, occurrence of nasal polyp, bi-laterality of nasal polyp) [25,34,35,38,39,41,43-47,50,51,61]. The risk of bias (methodological quality) was assessed using the Newcastle–Ottawa scale.
Statistical analyses
Meta-analyses were conducted in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). When the extracted data were continuous, meta-analyses were performed using the standardized mean difference (SMD). As there are no standardized scales for evaluating the percentage or absolute count of eosinophils, total nonspecific IgE, endoscopic polyp score, symptom score, olfactory function, or CT score, we used this method to calculate effect sizes. All other analyses were OR analyses of the incidences of outcomes.
Heterogeneity was calculated with the I2 test: The I2 test describes the rate of variation across studies because of heterogeneity rather than probabilistic chance; the measure ranges from 0 (no heterogeneity) to 100 (maximum heterogeneity). All results are reported with 95% confidence interval (95% CI), and all P-values were two-tailed. When significant heterogeneity among outcomes was found (defined as I2 >50), the random-effects model, according to DerSimonian-Laird, was used. This model assumes that the true treatment effects in individual studies may be different from one another, and that these are normally distributed. Those outcomes that did not present a significant level of heterogeneity (I2 <50) were analyzed with the fixed-effects model. The fixed-effects model uses the inverse variance approach, and it is assumed that all studies come from a common population. In addition, Subgroup analyses were done as a means of investigating heterogeneous results, which involve splitting all the participant data into subgroups in order to make comparisons between them. Subgroup analyses were done for subsets of different diagnostic criteria (such as tissue eosinophilia and clinicoradiological or laboratory characteristics), and for subsets of cutoff values (such as ≥10 eosinophils/HPF, ≥70 eosinophils/HPF, or ≥10% eosinophils/total infiltrating cells. Sensitivity analyses were performed to determine the effects of individual studies on the overall meta-analysis results. We used a funnel plot and Egger’s test simultaneously to detect publication bias. The trim-and-fill method also was done to indicate the significance of publication bias as well as provide biasadjusted results.
RESULTS
Search and study selection
In total, 55 studies with 6,143 participants were included in the meta-analysis. The characteristics, diagnostic criteria, and outcomes of the studies included in the analysis are summarized in Table 1. Many studies targeted only patients with CRS with nasal polyps. The diagnostic criteria were ≥10% eosinophils/total infiltrating cells in 16 studies, ≥70 eosinophils/HPF in 15 studies, the JESREC score in 12 studies, and ≥10 eosinophils/HPF in 12 studies. The outcomes were CT scores in 40 studies, total non-specific IgE in 20 studies, percentage or absolute count of eosinophils in 18 studies, nasal symptomatic scores in 15 studies, and olfactory function in 10 studies. The quality (risk of bias) assessment of the studies is summarized in Supplementary Table 1.
Comparison of laboratory, clinical, and radiological findings between ECRS and non-ECRS
Several different diagnostic criteria and cutoff values were used to classify ECRS, including tissue eosinophilia (≥10 eosinophils/HPF, ≥70 eosinophils/HPF, or ≥10% eosinophils/total infiltrating cells) and the clinicoradiological laboratory score (JESREC score ≥11). The common clinical symptoms of ECRS may vary according to the diagnostic criteria used. A subgroup analysis of diagnostic criteria (clinicoradiological or laboratory characteristics) showed no significant differences in nasal symptom scores or CT scores (P>0.05) among the four diagnostic criteria. However, there were significant differences in the serum percentage and count of eosinophils (P<0.05) and olfactory dysfunction (P<0.05). In post hoc analyses, serum eosinophil levels were significantly higher in ECRS than in non-ECRS for all diagnostic criteria (percentage of eosinophils: P<0.001, eosinophil count: P=0.007), although the values varied by group. With regard to olfactory dysfunction, ECRS diagnosed by the JESREC criteria was associated with significant olfactory dysfunction (SMD=0.446 [95% CI, 0.109–0.783]), whereas ECRS diagnosed by tissue eosinophilia (≥70) tended to be associated with decreased olfactory function, although the effects were not statistically significant (0.587 [95% CI, –0.227 to 1.401]).
In laboratory findings, the eosinophil percentage (SMD=1.561 [95% CI, 1.329–1.794], P<0.001, Cochrane Q=3 41.30, P<0.001, I2=89.5%), eosinophil count (SMD=1.493 [95% CI, 1.134–1.852], P<0.001, Cochrane Q=327.33, P<0.001, I2=93.0%), and total IgE (SMD=0.359 [95% CI, 0.118–0.600], P=0.004, Cochrane Q=69.54, P<0.001, I2=77.0%) were significantly higher in ECRS than in non-ECRS (Fig. 2). Regarding the clinical findings, nasal symptom scores (SMD=0.382 [95% CI, 0.156–0.608], P=0.0009, Cochrane Q=65.86, P<0.001, I2=75.7%), olfactory dysfunction (SMD=0.416 [95% CI, 0.037–0.794], P=0.031, Cochrane Q=86.62, P<0.001, I2=88.5%), and endoscopic nasal polyp scores (SMD=0.581 [95% CI, 0.314–0.848], P<0.001, Cochrane Q=124.01, P<0.001, I2=83.9%) were significantly higher in ECRS than in non-ECRS (Fig. 3). As a radiological finding, the whole-sinus opacification score (SMD=0.824 [95% CI, 0.588–1.059], P<0.0001, Cochrane Q=368.13, P<0.001, I2=88.9%) was significantly higher in ECRS than in non-ECRS (Fig. 4). In particular, anterior ethmoid sinus (SMD=0.535 [95% CI, 0.351–0.720], Cochrane Q=3.89, P=0.421, I2=0.0%) and sphenoid sinus (SMD=0.431 [95% CI, 0.143–0.718], Cochrane Q=8.98, P=0.062, I2=55.5%) opacification was significantly higher in ECRS than in non-ECRS (Supplementary Fig. 1). However, there were no significant differences in the frontal sinus (SMD=0.385 [95% CI, –0.065 to 0.835], Cochrane Q=30.42, P<0.001, I2=83.6%), maxillary sinus (–0.077 [95% CI, –0.410 to 0.256], Cochrane Q=17.52, P=0.004, I2=71.5%), ostiomeatal unit (SMD=0.241 [95% CI, –0.068 to 0.551], Cochrane Q=15.52, P=0.008, I2=67.8%), or posterior ethmoid sinus (SMD=0.454 [95% CI, –0.217 to 1.126], Cochrane Q=48.27, P<0.001, I2=91.7%) (Supplementary Fig. 2).

Forest plot. (A) Percentage of eosinophils, (B) Eosinophil count and (C) total immunoglobulin E in eosinophilic and non-eosinophilic chronic rhinosinusitis. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; HPF, high-power field.
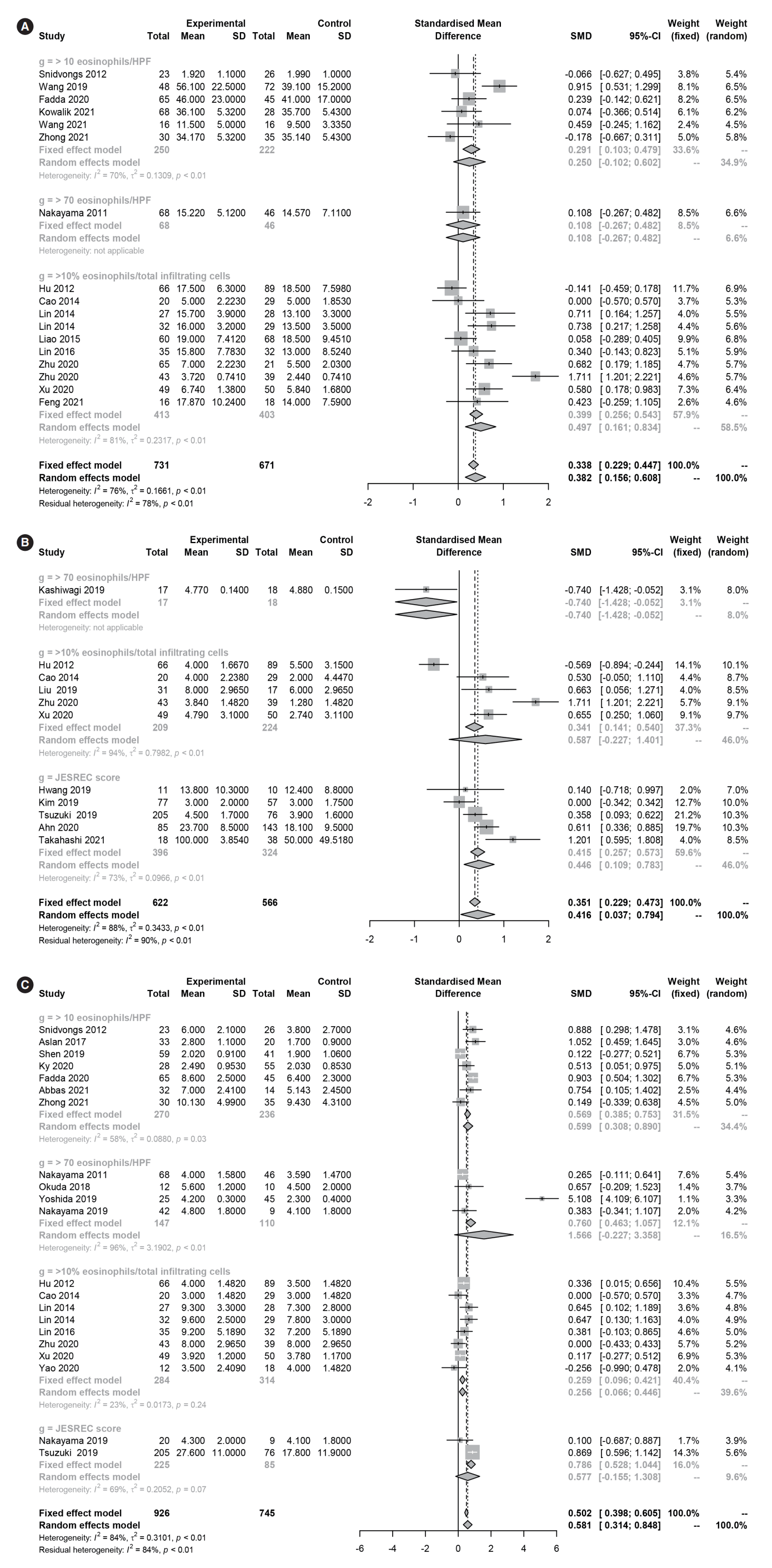
Forest plot. (A) Nasal symptom scores and (B) olfactory dysfunction in eosinophilic and non-eosinophilic chronic rhinosinusitis. (C) Endoscopic nasal polyp scores in eosinophilic and non-eosinophilic chronic rhinosinusitis. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; HPF, high-power field.

Whole-sinus opacification scores in eosinophilic and non-eosinophilic chronic rhinosinusitis. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; HPF, high-power field.
The Egger test and Begg funnel plot analyses for total IgE (P=0.600), nasal symptom scores (P=0.251), olfactory dysfunction (P=0.625), and endoscopic nasal polyp scores (P=0.302) revealed no publication bias in the included studies. However, the Egger test and Begg funnel plot analyses for eosinophil percentage (P=0.0004), eosinophil count (P=0.002), and the wholesinus opacification score on CT (P=0.008) suggested that some source of bias might have been included in this sample of studies. The Duval and Tweedie trim-and-fill method showed there was no significant difference between observed and adjusted values (percentage [1.561, P<0.001 vs. 0.946, P<0.001], count [1.493, P<0.001 vs. 0.847, P<0.001], whole-sinus opacification on CT [0.824, P<0.001 vs. 0.460, P=0.001]). Therefore, we concluded that the selected studies were not biased and that the results of these studies demonstrated the features associated with ECRS with respect to laboratory, clinical, and radiological findings. The funnel plot analysis results are provided in Supplementary Fig. 3. By contrast, Begg funnel plots and the Egger linear regression test for individual sinus lesions (for example, anterior ethmoid or posterior ethmoid opacification, etc.) were not conducted because of the small number of included studies (<10).
Comparison of comorbidities in ECRS versus non-CRS
Aspirin intolerance (SMD=4.657 [95% CI, 2.793–7.765], P<0.001, I2=0.0%), allergic rhinitis (SMD=2.008 [95% CI, 1.709–2.360], P<0.001, I2=21.0%), atopy (SMD=1.643 [1.315–2.053], I2=38.1%), and asthma (SMD=3.562 [95% CI, 3.042–4.170], P<0.001, I2=25.5%) showed significant associations with ECRS (Fig. 5A-D). Nasal polyp development (SMD=11.203 [4.721–26.587], P<0.001, I2=78.2%) and the occurrence of bilateral nasal polyps (SMD=5.510, [95% CI, 4.311–7.042], P<0.001, I2=28.8%) were also significantly associated with ECRS (Fig. 5E and F). A subgroups analysis according to the different diagnostic criteria showed no significant differences in comorbidities and nasal polyps (P>0.05).

Comparison of comorbidities between eosinophilic and non-eosinophilic chronic rhinosinusitis. (A) Aspirin intolerance. (B) Allergic rhinitis. (C) Aatopy. Comparison of comorbidities between eosinophilic and non-eosinophilic chronic rhinosinusitis. (D) Asthma. (E) Presence of nasal polyp. (F) Presence of bilateral nasal polyps. OR, odds ratio; CI, confidence interval.
Sensitivity analyses
We evaluated differences in pooled estimates by repeating the meta-analysis, each time omitting a different study. All results were consistent with the above results.
DISCUSSION
CRS, which involves inflammation of the nasal mucosa and sinuses, may result from various heterogeneous mechanisms. It thus may give rise to different clinical features in patients and require different treatment methods [3,38]. For example, ECRS is related to several conditions, including allergic rhinitis, asthma, aspirin sensitivity, and atopy [1]. In addition, ECRS has a strong tendency to recur after endoscopic sinus surgery and does not respond well to macrolide treatment, although it responds to systemic steroid treatment [2]. Therefore, accurately defining the CRS endotype can help establish a treatment plan, predict the prognosis, and identify possible comorbidities [62]. At present, histological confirmation is the gold standard for classifying ECRS and non-ECRS [55]. However, a uniform histological standard for diagnosing ECRS has not yet been established [1]. Most studies in this meta-analysis used the histological eosinophil count alone (including the percentage) to classify CRS as eosinophilic or non-eosinophilic. Different cutoff values were applied to define ECRS using tissue eosinophilia, including eight criteria for absolute eosinophil count (≥5, ≥8, ≥10, ≥50, ≥55, ≥70, ≥100, and ≥120 eosinophils per HPF [×400]), four criteria for the percentage of eosinophils/inflammatory cells (≥10%, ≥27%, ≥50%, and predominant), and one criterion based on clinicoradiological findings with the serum eosinophil score (JESREC score ≥11). However, some cutoff values were used in only one or two studies, and their potential for bias made it difficult to perform a meta-analysis and merge them with other cutoff values. Therefore, we selected cutoff values of ≥10 and ≥70 eosinophils per HPF for tissue eosinophilia and ≥10% for the percentage of tissue eosinophils for the analysis. We also included the JESREC score, which relies on clinical information, radiological findings, and the serum eosinophil count instead of a histological examination to diagnose ECRS.
In this study, serum eosinophils, IgE levels, nasal symptom scores, and endoscopic nasal polyp scores were significantly higher in ECRS than in non-ECRS. Eosinophils generate several cytotoxic mediators, including eosinophil peroxidase, which is produced under conditions of oxidative stress. These cells can cause serious damage to the epithelium and also play an important role in the pathogenesis of nasal polyps [63]. The amount of free radicals within nasal polyps is also associated with the severity of polyps [64]. Moreover, Th2-mediated inflammation, a major mechanism in ECRS, has also been demonstrated in asthma, allergic rhinitis, nasal polyps, and aspirin sensitivity, which may explain their close association [65-67].
Clinical symptoms may vary in the CRS patient population, and our study shows significant differences in olfaction between ECRS and non-ECRS. These results are similar to those of previous studies [27,30,40] that noted reduced olfaction in patients with ECRS. In this study, patients with ECRS had more bilateral lesions and nasal polyps than patients with non-ECRS. Therefore, we infer that olfaction was affected by the more severe lesions around the ethmoid cells of the olfactory epithelium in ECRS [42].
Significant differences were found in the level of olfactory dysfunction according to the diagnostic criteria used in studies. Two histological criteria (≥70% and ≥10%) did not reveal poorer olfactory function in ECRS than in non-ECRS. One criterion (≥70%) was used in only a single study, meaning that the results of this subgroup do not represent the true outcome. All studies using the other criterion (≥10%) used a visual analog scale (VAS), whereas most studies using the JESREC criteria used olfactory function tests, such as the butanol threshold test, T&T test, or KVSS II. Since VASs are weakly or moderately correlated with various olfactory function tests, asking patients about olfactory function would at best provide a rough diagnosis of anosmia versus normosmia that would not be reliable [68]. Similarly, in an analysis of the general population of Taiwan, only a weak correlation was found between olfactory function evaluated with various olfactory measurement tools and with a simple self-assessment [69].
Several reports have shown that CT can be useful for diagnosis, in particular in the early stage of ECRS. In ECRS, polyps and mucosal edema often appear around the middle turbinate on CT, which mainly corresponds to the ethmoid sinuses [4,12]. Furthermore, ECRS showed significantly higher rates of opacification of ethmoid and sphenoid sinuses (in particular anterior ethmoid sinuses) on CT than non-ECRS. Since sphenoid sinus lesions cannot be explained by the hypothesis of mucosal edema around the middle turbinate, additional studies are needed to clarify what this finding represents.
Subgroup analyses according to the different diagnostic criteria, including histological findings and clinicoradiological scores, showed significant differences in serum percentages and counts of eosinophils between ECRS and non-ECRS determined using all four diagnostic criteria. The mean peripheral eosinophil count tended to be significantly higher in patients with high mucosal eosinophil counts than in those with low counts. In general, the amount of eosinophils in the blood is representative of the amount of eosinophils in tissue [59]. Therefore, although the criteria had different cutoff values for tissue eosinophils, it is understandable that overall, higher levels of serum eosinophils correspond to ECRS.
To date, histological criteria have been the gold standard for diagnosing ECRS. In our study, several clinical findings, CT findings, and high peripheral eosinophil levels were also confirmed in patients diagnosed with ECRS. Therefore, it may be of clinical benefit to use clinicoradiological findings and/or a peripheral eosinophil scoring system applicable in outpatient clinics where histological testing of tissue eosinophilia is difficult.
This meta-analysis has several limitations. First, the data were collected from a limited number of regions (45 of 55 studies were from East Asia and South Asia), and geographic and genetic factors may influence the clinical characteristics of ECRS. Second, techniques for counting cells per HPF are currently not well standardized. Thus, the results may vary depending on the number of slides used, the level of magnification, the size of the HPF, and the tissue distribution pattern of eosinophils [70]. In addition, since the treatment outcomes were heterogeneous across studies, the diagnostic criteria may have varied accordingly. Therefore, it is possible that the reason for using different diagnostic criteria for ECRS was to more prominently identify outcomes such as specific sinonasal quality of life, recurrence, and the effects of drugs. Third, bias could have been introduced by the medications used prior to clinical manifestations and assessment of eosinophils, because blood and tissue eosinophil counts and nasal polyp size decreased following the initiation of systemic steroid therapy and similar levels were maintained several weeks after the discontinuation of steroids [71-73]. Therefore, clinicians should consider the patient’s history of drug treatment when diagnosing ECRS [70]. This may explain some of the heterogeneity in our results. To overcome these limitations and increase the accuracy of the clinical classification of ECRS, it is necessary to standardize technical issues, such as the counting of eosinophils for a conventional ECRS diagnosis, detailed clinical examinations, and meticulous recording of the patient’s medication history.
In conclusion, several clinical characteristics, such as higher endoscopic polyp and nasal symptom scores and elevated serum eosinophil counts, are associated with ECRS. Furthermore, sinus opacification (in particular the anterior ethmoid or sphenoid sinuses) on CT is also associated with ECRS. Therefore, if it is difficult to conduct histological examinations to diagnose ECRS, criteria such as the JESREC score could be used.
HIGHLIGHTS
▪ The histological and clinical criteria showed similar trends in nasal symptoms, computed tomography (CT), and serum eosinophil levels.
▪ Higher endoscopic polyp and nasal symptom scores and elevated serum eosinophil counts are associated with eosinophilic chronic rhinosinusitis (ECRS).
▪ Opacification of the anterior ethmoid or sphenoid sinuses on CT is associated with ECRS.
▪ When it is difficult to diagnose ECRS on an outpatient basis, clinical and laboratory features can be used.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Ministry of Science & ICT (2018M3A9E8020856, 2019M3A9H2032424, 2019M3E5D5064110).
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: DHK, SHH. Data curation: SWK, MAB. Formal analysis: SHH, DHK. Funding acquisition: DHK, SWK. Methodology: SWK, MAB; Project administration: DHK, SHH. Visualization: SWK, MAB. Writing–original draft: DHK, SHH. Writing–review & editing: all authors.
Supplementary Materials
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2022.00052.
Quality (risk of bias) assessment
Sinus opacification scores in eosinophilic and non-eosinophilic chronic rhinosinusitis. (A) Anterior ethmoid sinus. (B) Sphenoid sinus. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval.
Sinus opacification scores in eosinophilic and non-eosinophilic chronic rhinosinusitis. (A) Frontal sinus. (B) Maxillary sinus. (C) Ostiomeatal unit. (D) Posterior ethmoid sinus. SD, standard deviation; SMD, standardized mean difference; CI, confidence interval; JESREC, Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis.
Begg funnel plots. (A) Serum immunoglobulin E. (B) Nasal symptom scores. (C) Olfactory dysfunction. (D)Endoscopic nasal polyp scores. (E) Serum eosinophil percentage. (F) Serum eosinophil count. (G) Whole-sinus computed tomography score.


