INTRODUCTION
Allergic rhinitis is a chronic allergic inflammatory disease of the nasal cavity with typical symptoms of nasal obstruction, watery rhinorrhea, sneezing and itching [1]. It is a global health problem in the modern era, with a 10%ŌĆō30% prevalence rate throughout the world [2]. Early diagnosis and adequate intervention are mandatory to improve quality of life and to prevent further sensitization to other allergens and development of allergic asthma [3,4].
Nasal provocation testing (NPT) can be used to evaluate the actual response of the nasal mucosa after direct exposure to causative allergens into the nasal cavity [5]. NPT is quite useful for diagnosing allergic rhinitis [6] as well as local allergic rhinitis (LAR) [7,8]. In spite of its usefulness, many practitioners still are not performing NPT because of its time-consuming process and variability of results according to many factors such as examiner and concentration of allergen. Therefore, it is so essential that standard protocol for NPT with fixed allergen dosage should be developed.
For the NPT standard protocol, a standardized provocative agent with a fixed allergen dose and formula are needed. Until recently, house dust mite (HDM) extract for NPT was produced by Allergopharma (Hamburg, Germany) [2,9]. However, they have stopped producing it about 1 year ago. Recently, Lofarma (Milan, Italy) started to produce a new HDM extract, Allerkin (Lofarma), for use as an NPT agent. However, no studies have been done to evaluate its usefulness for diagnosing allergic rhinitis by NPT.
Therefore, we aimed to evaluate the clinical usefulness of Allerkin in patients with rhinitis symptoms by evaluating changes in nasal symptoms and acoustic parameters after exposure to HDM extract.
MATERIALS AND METHODS
Patients
Twenty patients (16 males and 4 females, mean age: 29.6┬▒14.6 years) who suffered from typical symptoms of rhinitis for more than a year were enrolled in this study. We performed skin prick tests (SPTs) for all patients using more than 40 aeroallergens including HDM (Dermatophagoides pteronyssinus and D. farinae), animal dander (dog and cat), cockroaches, and grass/tree/weed pollens. Detailed inclusion and exclusion criteria are summarized in Table 1. This study was approved by the Institutional Review Board (IRB No. 15-007) of Inha University College of Medicine.
Nasal provocation test (NPT) using Allerkin test and acoustic rhinometry
Patients were acclimatized in a room at regular temperature (approximately 20┬░C) and relative humidity (about 50%) for 15 minutes prior to beginning the study. Before allergen provocation, patients were asked about their baseline symptoms, including nasal obstruction, rhinorrhea, sneezing and itching (using a visual analogue scale [VAS] questionnaire). We also performed acoustic rhinometry to evaluate the volume and dimension of the nasal cavity. Minimal cross-sectional area (MCA) was defined as the cross-sectional area with the smallest dimension within the nose. Total nasal volume (TNV) was calculated as the summation of all cross-sectional areas from the nostril to 7 cm deep in the nasal cavity.
To rule out nonspecific nasal hyper-reactivity, we first performed challenge using lactose powder (Lofarma) as a control. Using a specially designed device provided by the company, the lactose powder was delivered directly into the nose. Fifteen minutes after control challenge, the VAS questionnaire and acoustic rhinometry were repeated. If there a more than 29% decrease of TNV and/or MCA from the baseline value, we determined that he or she had nonspecific hyper-reactivity and the patient was disqualified from the study. For patients without nonspecific hyper-reactivity, we performed allergen challenge (40 allergic units of Dermatophagoides mix powder in a capsule) using the same delivery device. Fifteen and 30 minutes after allergen challenge, the VAS questionnaire about nasal symptoms and acoustic rhinometry were repeated.
We defined the change in nasal symptoms as (post-NPT VAS score) minus (pre-NPT VAS score). We also defined the total nasal symptom score (TNSS) as the sum of all nasal symptoms. For acoustic parameters (TNV and MCA), we used the following formula to evaluate the percentage decrease from the baseline value:
RESULTS
Patients were categorized into three groups. Patients in group A (the nonallergic group, n=8) showed negative results for all tested aeroallergens in SPT. After NPT challenge using Allerkin, they also had non-provocative results (a less than 25% decrease of TNV and MCA from the baseline value). Patients in group B (the allergic group, n=7) showed strongly positive results (a wheal size larger than that of histamine) for HDM allergens. Finally, patients in group C (the local allergic group) showed negative results on SPT, but a provocative response in NPT (a more than 29% decrease in TNV/MCA from the baseline value). There were no significant differences in characteristics of patients in sex, age, and disease severity according to the Allergic Rhinitis and its Impact on Asthma 2008 guidelines.
Compared to group A, patients in groups B and C tended to suffer from more aggravated nasal symptoms after HDM challenge. Patients in group C showed significant aggravation of nasal obstruction compared to group A (P<0.05) (Fig. 1).
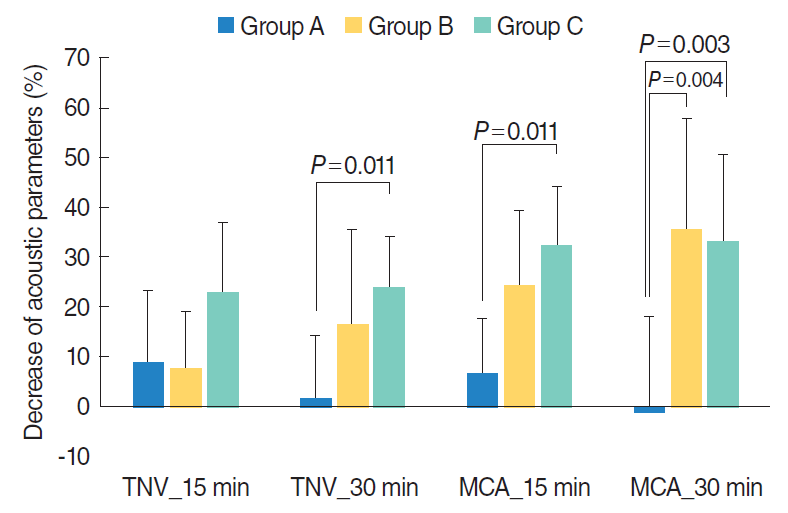
Patients in groups B and C showed a greater decrease in TNV and MCA compared to group A. Thirty minutes after HDM challenge, groups B and C showed significantly greater decreases in MCA compared to group A (P<0.01) (Fig. 2).
DISCUSSION
NPT has many advantages over SPT in clinical practice. First of all, NPT could ŌĆśdefinitelyŌĆÖ evaluate the hyper-responsiveness of the ŌĆśnasal cavity itself,ŌĆÖ after the actual allergen challenge. In some patients for whom we clinically suspect allergic rhinitis, the result of SPT could be negative. In other patients, result for SPT is strongly positive to multiple allergens. In these cases, NPT could be useful in diagnosing LAR and differentiating clinically important allergen. Furthermore, NPT could also be useful before initiating immunotherapy. If a patient shows positive result for NPT, practitioners could be sure that allergen used for NPT is the actual provocative allergen for that patient and initiate immunotherapy.
NPT is done to evaluate the actual response of the nasal cavity after exposure to causative aeroallergens. We previously reported the usefulness of NPT in diagnosing allergic rhinitis [6] and proposed diagnostic criteria of allergic rhinitis using NPT [9]. Until recently, the only available HDM extract for NPT was produced by Allergopharma. However, they stopped producing it, making it essential to establish a new protocol for NPT using a different HDM extract.
Allerkin is a new, mostly untested product for NPT. A literature search for Allerkin yielded only 3 published papers, all of which were about intranasal immunotherapy agents [10-12]. Therefore, to the best of our knowledge, this is the first study testing the usefulness of Allerkin as an agent for NPT. Allerkin sells both 20 AU and 40 AU HDM extract capsules. However, in our preliminary study, we observed that 20 AU was not enough for provocative use. Therefore, we used only 40 AU in our present study.
The main difference between Allerkin and Allergopharma HDM extract is that the former is a dry powder while the latter is dissolved in a liquid solvent. Using a specially designed delivery device (Lofarma), we delivered dry powder directly into the nasal cavity. The advantage of dry powder is that we were able to minimize nonspecific hyper-reactivity caused by any solvent. In our present study, to rule out nonspecific hyper-reactivity caused simply by dry powder, we tested provocation with a control powder (lactose) instead of normal saline. None of the patients had nonspecific hyper-reactivity caused by lactose powder. Interestingly, a large proportion of patients showed nonspecific hyper-reactivity provoked by challenge with normal saline only.
LAR is defined as an absence of systemic allergic sensitization and localized allergic reaction of the nasal cavity [13,14]. When it was first diagnosed by Huggins and Brostoff [15], they suggested that it be detected via elevated specific IgE in the nasal secretions of patients. However, as the sensitivity of detecting specific IgE in nasal secretions is quite low (about 22% to 40%) [7,16], NPT was proposed as an alternative for diagnosing LAR. For example, if a patient has a negative result on SPT and a provocative result on NPT, we could diagnose that patient as having LAR [7]. In our previous study, we suggested that NPT with acoustic rhinometry was useful for the differential diagnosis of LAR [17]. In our present study using Allerkin, we observed that among 15 patients with negative SPT results, 7 patients had provocative responses on NPT and fell into group C (local allergic group). Therefore, we suggest that the new NPT agent, Allerkin, may be useful for the diagnosis of LAR.
Evaluation of symptom changes after allergen provocation is a reasonable procedure for NPT. Rondon et al. [7] suggested more than 30% symptom aggravation from baseline as the diagnostic criteria. Our diagnostic criteria for LAR were a nasal obstruction score >2, and a TNSS aggravation score >6.5 [17]. In the present study, the mean TNSS change of groups B and C was greater than that of group A (group A, 2.0┬▒2.3; group B, 3.4┬▒2.6; group C, 3.4┬▒1.9). Further studies with more patients are necessary to prove this tendency with more significance and relevance.
The use of acoustic rhinometry to measure the dimensions and volume of nasal cavity is based on sound reflection. Acoustic rhinometry has many advantages; it is noninvasive, it requires only minimal cooperation from the patient, it is not time consuming, and it is reproducible when performed by an experienced examiner [9]. The most widely accepted criterion for positive result is a more than 29% decrease in MCA from the baseline value (NPT 23, 24), as healthy, symptom-free individuals could have a 10%ŌĆō15% MCA decrease after provocation [18]. In our present study, 30 minutes after provocation using Allerkin HDM extract, groups B and C had significant decreases of MCA (group B, 35.4%┬▒22.5%; group B, 33.1%┬▒17.6%), while group A showed almost no change (ŌĆō1.1%┬▒19.3%). Therefore, we suggest that Allerkin HDM extract is useful for diagnosing allergic rhinitis using NPT.
One of the limitations of our study is that only a small number of patients were enrolled. As Allerkin HDM extract is still not commercially available in Korea, we performed the study with noncommercial products purchased by research funding. When the product could be imported in Korea for NPT, we could enroll much more patients to evaluate its usefulness in future study. Further study with more patients could yield more meaningful results.
Measuring the aggravation of symptoms after NPT, we found significant difference in only nasal obstruction (but no other symptoms). In our previous studies for NPT using Allergopharma HDM extract, patient with allergic rhinitis exhibited significant aggravation of all symptoms [9]. The main reason for this discrepancy between former study and this one is the number of patients enrolled. In our previous study, we enrolled 208 patients and another 222 control group [9]. However, in this study, we could only enroll 20 patients. Further study with more patients could yield more meaningful difference.
One could argue that Allerkin is just another formulation of HDM extract and is nothing new at all. However, for clinical practice, standard protocol using fixed methodology and allergen dosage is quite important for the reproducibility of results, and comparison of results between researchers. As Allerkin is the only commercially available allergen extract for now, it is important to set up a standard protocol to share with other researchers.
Regarding the concentration, we first tried on 20 AU and 40 AU. However, there was absolutely no significant difference between patient and control group using 20 AU. Therefore, we decided to use 40 AU only. Surely, we could get more data if we could use different concentration of HDM extract. On the other hand, it would be quite time-consuming. In fact, NPT using single allergen concentration needs about 40 minutes. If we use several concentration of allergen, we need several hours for examination and it would not be good for clinical everyday practice.
In conclusion, Allerkin HDM extract may be a useful provocative agent in NPT for diagnosing allergic rhinitis and LAR.