 |
 |
- Search
AbstractObjectivesMastoid obliteration is used to obliterate the mastoid cavity following a mastoidectomy or to prevent the formation of a retraction pocket. This study evaluated the effectiveness of ╬▓-tricalcium phosphate and polyphosphate (╬▓-TPP) for mastoid obliteration in middle ear surgeries in prospective human and animal studies.
MethodsTwenty patients with chronic otitis media underwent mastoid obliteration using ╬▓-TPP after a intact canal wall mastoidectomy or simple mastoidectomy. The clinical data were prospectively evaluated including: the diagnosis, temporal bone computed tomography (TBCT), otoscopic findings, pure tone audiogram, and complications. In the animal experiment, ╬▓-TPP was applied into the right bulla in five rats, and the opposite bulla was used as the control in the non-obliterated state. The skulls of five other rats were drilled out and the holes were obliterated with ╬▓-TPP. TBCT were obtained at 3, 6, and 9 months after the obliteration and histologic analysis was done at 3 and 9 months after surgery.
ResultsIn the human study, fourteen TBCTs were obtained at 12 months after the surgery. All demonstrated no bone resorption in the obliterated mastoids. Among the 15 cases displaying retracted tympanic membranes preoperatively, 11 showed no retraction, 2 showed retraction postoperatively, 1 was lost to follow-up and 1 was a case of postoperative wound infection. Among 20 cases, one case developed a postoperative infection that necessitated a second operation. Sixteen underwent ossiculoplasty; hearing improvements were obtained in 15 cases and 1 case showed decreased hearing. In the animal study, new bone formation without significant bone resorption in the radiologic and histologic findings were noted in both the skull and bulla groups.
A mastoidectomy is performed as a surgical method to completely remove inflammatory tissues or cholesteatoma and to prevent recurrence in chronic otitis media (COM) patients. The procedure can be classified as a canal wall down mastoidectomy (CWDM, also known as open cavity mastoidectomy [OCM]) or canal wall up mastoidectomy (CWUM). In the case of CWDM, it is possible to remove the lesion completely by securing a sufficient surgical view. However, the disadvantages of CWDM include cavity problems, such as otorrhea and dizziness, and difficulty in wearing hearing aids after surgery. On the other hand, CWUM does not appreciably change the anatomical structure, however the recurrence rate is higher than CWDM because of the limited surgical view. In addition, a retraction pocket may develop if the Eustachian tube function is insufficient in CWUM [1,2]. To compensate for these disadvantages of CWDM, reconstruction of the posterior wall of the external auditory canal (EAC) and mastoid obliteration have been attempted, especially when the mastoid cavity volume is too large to prevent a cavity problem. In CWUM, obliterating the epitympanum and mastoid cavity is performed to prevent the retraction pockets [2,3].
Autografts, such as bone, cartilage, bone pate, abdominal fat, and musculoperiosteal flap have been used for a long time as the ideal materials of the graft for mastoid obliteration. However, each has disadvantages; the amount of cartilage or bone pate is insufficient, a large amount of fat is resorbed, and the length of the musculoperiosteal flap is short. Therefore, the mastoid cannot be obliterated completely and the obliteration materials gradually atrophies [4,5]. To compensate for these limitations, xenografts or artificial materials such as hydroxyapatite have been tried [6].
╬▓-Tricalcium phosphate and polyphosphate (╬▓-TPP) is a novel artificial bone. It has been used in orthopedic surgery (e.g., artificial hip joint surgery, spine surgery), plastic surgery (e.g., craniofacial plastic surgery), and dental surgery (e.g., dental implant surgery), indicating its usefulness as a substitute bone graft material.
The aim of this prospective study was to analyze the treatment efficacy of mastoid obliteration using ╬▓-TPP after CWUM in patients who showed poor gas exchange ability but for whom CWDM was inappropriate. Additionally, its efficiency was also evaluated in animal models.
This study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-DEV-DE4-09-263). Twenty patients who underwent a mastoid obliteration with powder type PolyBone┬« (╬▓-TPP; Kyoungwon Medical Co., Seoul, Korea) during a mastoidectomy between January 2010 and March 2011 were enrolled in this study. They were followed up for a minimum of 1 year. All patients received a clear explanation about a mastoid obliteration using PolyBone┬« and had provided their written consent. This study was limited to patients with cholesteatoma showing the possibility of retraction pockets or recurrence after operations. Several cases with COM which showed poor gas exchange ability with contracted mastoid cavities or cicatrical mucosa were also included in this study. Patients were excluded if they were Ōēż5 years-of-age, displayed abscess formation or intracranial problems as complications of COM, had continuous otorrhea and had an ear discharge culture study that confirmed antibiotic-resistant bacteria, or if they disagreed with the aim or method of the study.
All surgeries were performed by the same surgeon. Under general anesthesia with a retroauricular approach, the Palva flap was elevated and the cortical bone pate was harvested from the intact, non-infected portions and mixed with antibiotics before the mastoidectomy. After removing the inflamed tissues completely by CWUM (intact canal wall mastoidectomy [ICWM] or simple mastoidectomy [SM]), 5 g of powder type PolyBone® was mixed with 2 mL of normal saline. First, the space between the attic and the antrum was blocked with gelfoam, and care was taken not to spread to the epitympanum. Then, the mastoid cavity was carefully filled with prepared PolyBone®, avoiding the invasion of the epitympanum. PolyBone® was completely covered by the harvested bone pate (Fig. 1). Finally, the flap was covered and subcutaneous tissue and skin were sutured layer-by-layer.
The EAC and tympanic membrane (TM) condition, retraction pocket or cholesteatoma recurrence, and complications were investigated and recorded by endoscopic and microscopic examination at 1, 2, and 4 weeks, and 3, 6, 9, and 12 months after surgery. To compare with preoperative hearing, pure tone audiometry (PTA) was performed at 3 and 12 months after surgery. The hearing threshold was measured by an average of 0.5, 1, 2, and 3 kHz according to the 1995 AAO-HNS guidelines [7]. The degree of resorption of PolyBone® and new bone formation were analyzed for a follow-up using temporal bone computed tomography (TBCT) that obtained at 12 months after surgery.
Ten, 7- to 8-week-old, female Sprague-Dawley rats showing normal EAC and middle ear by optical microscopic examination were used in this animal study. Animal care, housing, and experimental procedures were conducted according to the guidelines for animal experiments of Ajou University. In the laboratory, day and night cycles were maintained constantly and the rats were raised with a commercial pellet feed.
Skull or bulla obliteration with PolyBone┬« was performed in 10 rats (n=5 in the skull group, n=5 in the bulla group). Preoperative anesthesia was achieved using a mixture of Ketamin (50 mg/kg) and Xylazine (5 mg/kg) by injection in the peritoneal space. Using a drill, 4├Ś4 mm sized holes were made on the right and left side of the skull of five rats under surgical microscope (OPMI Pico; Carl Zeiss, Oberkochen, Germany); the right holes were obliterated by PolyBone┬« and the left holes were not obliterated as a control. The subcutaneous tissue and the skin above both holes were sutured. For the other five rats, incisions on the anterior midline neck were made to expose both sides of the bullae (anterior approach) and, after making 3├Ś3 mm sized holes on the bulla using a drill, all mucosa inside the bulla were cauterized with 10% trichloroacetic acid. Obliteration with PolyBone┬« and suturing were done for the right side bulla, with only suturing done for the left side.
Coronal plane CT images were acquired 3, 6, and 9 months after the surgery, and the results from the treated right side and the control left side were compared. After anesthesia, the animals were quickly decapitated. The skulls and bullae were harvested from rats at 3 months (1 rat) and 9 months (4 rats) after the surgery for histologic analyses. Each harvested specimen was decalcified with ethylene diamine tetracyclic acid (EDTA) for a week. They were then embedded in paraffin and sliced into 12 ┬Ąm-thick sections. The slides were stained with hematoxylin and eosin (H&E) and observed using an optical microscope.
The flow diagram of human study can be seen in Fig. 2. Twenty patients (11 males, 9 female) underwent mastoid obliteration with PolyBone® during middle ear surgeries. The average age of the patients was 49.9±10.9 years (range, 19 to 66 years). Among these patients, 5 were diagnosed with COM, 13 with middle ear cholesteatoma, and 2 with cholesterol granuloma. Among the 13 patients with middle ear cholesteatoma, the detail diagnosis was attic cholesteatoma for 8, tensa retraction cholesteatoma for 3, sinus cholesteatoma for 1, and recurred attic cholesteatoma for 1. ICWM was performed for 16 patients and SM was performed for four patients; for one patient, the surgery was the second operation because of recurrent cholesteatoma, while being the first operation for the other 19 patients (Table 1). Of 15 patients who showed tympanic retraction preoperatively, 11 did not show retraction after the surgery, 2 displayed recurrent membrane retraction, 1 was lost to follow-up, and 1 was a case of postoperative wound infection. All 5 patients who showed no retraction before surgery also did not display a retraction postoperatively (Fig. 3).
For all 20 patients, preoperative TBCT images were taken and follow-up CT images 1 year after the surgery were acquired in 14 patients. Among the 14 patients with a follow-up CT, no patient showed a sign of bone resorption and maintained the mastoid obliteration status (Fig. 4). Among 6 patients without a follow-up TBCT, 2 were lost to follow-up, 1 got infected, and the other 3 refused a follow-up TBCT.
PTA before the surgery and 1 year after the surgery were compared, except for one patient who was lost to follow-up and one patient with postoperative infection. Among the 18 patients with follow-up PTA data, 15 patients showed improved hearing and all 15 were patients underwent ossiculoplasty with a partial ossicular replacement prosthesis (PORP). Two patients who underwent tympanization only without ossiculoplasty showed no change before and after surgery. The other one patient who underwent ossiculoplasty showed decreased hearing.
After the surgery, only one complication was evident in a COM patient who underwent SM and ossiculoplasty with PORP. In this patient, methicillin-resistant Staphylococcus aureus (MRSA) was cultured from the ear discharge before the surgery. However, the patient showed no otorrhea during the surgery (Fig. 5), and the mastoid obliteration was performed. Five days after the surgery, inflammation was evident at the operative site. Ten days after surgery, the PolyBone® filling of the mastoid cavity was removed and OCM was performed. After the second surgery, the patient was followed-up on an outpatient basis without complications.
On CT images taken 3, 6, and 9 months after the surgery, all 4 rats whose skull holes were filled with PolyBone® showed continuous bone tissue density inside the holes, which was indicative of bone formation without bone resorption. The other four rats with PolyBone® filled holes in the bulla also showed a similar pattern in CT images (Fig. 6). The right side, PolyBone® filled holes of the skulls showed new bone formation with osteocytes without signs of inflammation on H&E staining. The left, unfilled holes showed significantly less new bone formation than the right side 3 and 9 months postoperatively (Fig. 7). The right side holes in the bulla that had been filled with PolyBone® also showed new bone formation on the H&E staining.
CWUM is one of the surgical methods in COM patients that can maintain the original contour of the EAC. However, a retraction pocket may occur in patients who have poor Eustachian tube function due to negative pressure in the preserved air-reserved epitympanum and mastoid cavity. The more air-filled spaces there are, the greater the probability that a retraction pocket will occur [1].
Mastoid obliteration is used to prevent cavity problems in patients with large mastoids during CWDM, and to prevent the occurrence of a retraction pocket in CWUM. Temporalis muscle, autologous cortical bone pate, cartilage, abdominal fat, alloplastic materials, and biosynthetic materials have been introduced for use in mastoid obliteration [8-12]. The most commonly used material is the musculoperiosteal flap, which is easily designed in the same operation field and has a good prognosis [8,9]. The musculoperiosteal flap including pedicled flaps that include the anteriorly based Palva flap, inferior based flap, and Rambo flap can be used for mastoid obliteration [13-15]. These flaps have an advantage of resistance to infection. However, resorption and contraction can occur postoperatively [13-16]. A donor site defect and difficulty in harvesting large volumes are additional problems. Various artificial materials are being used to compensate for the problems of autografts [17-20].
Hydroxyapatite (HA) is a bioinert, alloplastic material comprised of a calcium phosphate ceramic. It has been used as an ossicular prosthesis material or for reconstruction of the posterior EAC wall in the otologic field [21]. As HA is a mineral matrix in bone tissue, it completely combines with bone tissue after being inserted in human bone. In one study, HA granules marketed as Bongros® (Bio@ Co., Seoul, Korea) were applied into the bulla of guinea pigs as a bone graft and there was successful substitution with normal new bone formation without inflammatory reaction or inner ear toxicity reported [22]. In another study, bone formation was induced by HA implantation in the temporal dorsal bulla of guinea pigs without inflammation after 1 year and there was no recurrence in a cholesteatoma patient after mastoid obliteration with HA granules 2 years postoperatively [23]. HA cement marketed as Mimix® (Walter Lorenz Surgical Inc, Jacksonville, FL, USA) has been used as a reconstruction material after the middle cranial fossa or posterior cranial fossa approach [24]. However, complications such as delayed osseointegration failure, infection, and severe osteitis have been documented after performing mastoid obliteration using HA cement, despite complete coverage with fascia, flap, and skin [25].
The PolyBone® used in this study is a new artificial bone comprised of β-TPP. It is available both in powder and granule form. PolyBone® contains an osteoinduction factor that stimulates the differentiation of osteoblasts and increases the expression of bone morphogenetic protein-4. The mechanism of stimulating bone formation involves participation of the polyphosphate in bone mineralization through the control of Ca concentration and pH in osteoblasts, which affects signal transduction to induce the stimulation of bone formation. In addition, PolyBone® has high biocompatibility without producing an inflammatory reaction. Synthetic materials with higher biocompatibility have been recently evaluated in animal experiments concerning orthopedic use. However, there have been few reports of the otologic clinical applicability of such materials [2,21,26].
PolyBone® has lower strength, similar to that of cancellous bone. It has an advantage in bone union because the absorption rate of the implanted bone and the rate of bone growth closely corresponds. Therefore, PolyBone® is frequently used in posterior lumbar interbody fusion or posterolateral fusion in orthopedic surgery and neurosurgery [27].
In one study, the granule type of PolyBone® was more effective than the powder type in the obliteration of bullae in guinea pigs [28]. They commented that the granule type produced relatively low inflammatory reactions and obvious new bone formation with biodegraded graft materials. On the other hand, the powder type caused serious inflammatory reactions with unresolved materials until 20 weeks after obliteration. Despite these observations, the powder type of PolyBone® was applied to all 20 patients in this study, because the mastoid area is not subject to load and because obliteration is performed on the premise that inflammation had completely been removed. The authors considered that it is more important to completely obliterate the mastoid cavity to prevent a TM retraction caused by negative pressure than to induce osseointegration between new bone formation and degradable synthetic materials in a short time.
The purpose of mastoid obliteration after CWUM is to prevent the recurrence of a retraction pocket in patients who showed poor gas exchange ability. In this study, patients without a retracted TM preoperatively did not show a retracted TM postoperatively. Among the 15 cases which had a retracted TM preoperatively, 13 cases were followed-up. Eleven cases (73.3%) showed no retraction postoperatively. All fourteen TBCT obtained at 12 months after surgery demonstrated no bone resorption in obliterated mastoids. Pre- and postoperative hearing tests produced various results depending on whether ossiculoplasty had been performed or not. In the animal experiments, new bone formation was observed around obliterated PolyBone® without inflammatory cells and showed successful osseointegration.
PolyBone®, which was developed as a new biomaterial substitute for bone implantation, produces nearly no inflammatory reaction due to high biocompatibility. However, we confined candidates of mastoid obliteration with PolyBone® to patients without otorrhea and infection, due to the possibility of a foreign body reaction to alloplastic materials. During the operations, we collected an autologous bone pate from a non-inflammed mastoid cortex bone before a mastoidectomy. In all cases, we covered the autologous bone pate thoroughly over obliterated PolyBone® with care not to contact with patient's subcutaneous tissue to reduce the possibility of foreign body reaction. In one patient with complications, MRSA was isolated preoperatively from otorrhea culture. Mastoid obliteration using PolyBone® was performed, because no inflammatory sign was shown in intraoperative findings, except for a slightly wet middle ear mucosa. However, erythema and swelling occurred on postoperative day 5 and PolyBone® was removed on postoperative day 10. This experience highlights the importance of avoiding this technique in infected conditions, especially in a wound infected with antibiotic resistant bacteria. The remaining 19 patients have been followed-up in the outpatient clinic without postoperative complications, such as infection or foreign body reaction. However, surgeons must keep in mind the possibility of minor and major infection or foreign body reaction during performing mastoid obliteration with PolyBone®.
Mastoid obliteration is one of the effective options to prevent ear TM retraction after middle ear surgeries for patients with poor gas exchange ability. Although long-term follow-up data are needed, PolyBone® can be a relatively safe and effective material in mastoid obliteration in a COM operation when patients are accurately selected that have no inflammation or otorrhea, and by monitoring during the performance of the procedure to cover PolyBone® with an autologous bone pate to avoid exposure to surrounding subcutaneous soft tissue.
NotesThis study was presented at 86th Congress of Korean Society of Otorhinolaryngology-Head and Neck Surgery, April 29, 2012. References1. Palva T, Virtanen H. Ear surgery and mastoid air cell system. Arch Otolaryngol. 1981 2;107(2):71-73. PMID: 7469894.
2. Lee WS, Choi JY, Song MH, Son EJ, Jung SH, Kim SH. Mastoid and epitympanic obliteration in canal wall up mastoidectomy for prevention of retraction pocket. Otol Neurotol. 2005 11;26(6):1107-1111. PMID: 16272924.
3. Palmgren O. Long-term results of open cavity and tympanomastoid surgery of the chronic ear. Acta Otolaryngol. 1979;88(5-6):343-349. PMID: 532609.
4. Merifield DO. Obliteration of the mastoid segment: a clinical review and pilot study of various transplant materials. Ann Otol Rhinol Laryngol. 1963 3;72:157-190. PMID: 13934989.
5. Solomons NB, Robinson JM. Obliteration of mastoid cavities using bone pate. J Laryngol Otol. 1988 9;102(9):783-784. PMID: 3171368.
6. Lenis A. Hydroxylapatite canal wall reconstruction in patients with otologic dilemmas. Am J Otol. 1990 11;11(6):411-414. PMID: 2178321.
7. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995 9;113(3):186-187. PMID: 7675477.
8. Palva T. Operative technique in mastoid obliteration. Acta Otolaryngol. 1973 4;75(4):289-290. PMID: 4702622.
9. Gantz BJ, Wilkinson EP, Hansen MR. Canal wall reconstruction tympanomastoidectomy with mastoid obliteration. Laryngoscope. 2005 10;115(10):1734-1740. PMID: 16222186.
10. Shea MC Jr, Gardner G Jr, Simpson ME. Mastoid obliteration using homogenous bone chips and autogenous bone paste. Trans Am Acad Ophthalmol Otolaryngol. 1972;Jan-Feb;76(1):160-172. PMID: 4337026.
11. Black B. Mastoidectomy elimination: obliterate, reconstruct, or ablate? Am J Otol. 1998 9;19(5):551-557. PMID: 9752959.
12. Dornhoffer JL. Surgical modification of the difficult mastoid cavity. Otolaryngol Head Neck Surg. 1999 3;120(3):361-367. PMID: 10064639.
13. Meurman Y, Ojala L. Primary reduction of a large operation cavity in radical mastoidectomy with a muscle-periosteal flap. Acta Otolaryngol. 1949 6;37(3):245-252. PMID: 15396404.
14. Palva T, Palva A, Karja J. Musculoperiosteal flap in cavity obliteration: histopathological study seven years postoperatively. Arch Otolaryngol. 1972 2;95(2):172-177. PMID: 5060065.
15. Thomas Rambo JH. Further experience with musculoplasty. AMA Arch Otolaryngol. 1960;71(3):428-436.
16. Cho SW, Cho YB, Cho HH. Mastoid obliteration with silicone blocks after canal wall down mastoidectomy. Clin Exp Otorhinolaryngol. 2012 3;5(1):23-27. PMID: 22468198.
17. Minoda R, Hayashida M, Masuda M, Yumoto E. Preliminary experience with beta-tricalcium phosphate for use in mastoid cavity obliteration after mastoidectomy. Otol Neurotol. 2007 12;28(8):1018-1021. PMID: 17898672.
18. Goldenberg RA. Hydroxylapatite ossicular replacement prostheses: preliminary results. Laryngoscope. 1990 7;100(7):693-700. PMID: 2163478.
19. Grote JJ. Reconstruction of the ossicular chain with hydroxyapatite implants. Ann Otol Rhinol Laryngol Suppl. 1986;Mar-Apr;123:10-12. PMID: 3083755.
20. Estrem SA, Highfill G. Hydroxyapatite canal wall reconstruction/mastoid obliteration. Otolaryngol Head Neck Surg. 1999 3;120(3):345-349. PMID: 10064636.
21. Ambard AJ, Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont. 2006;Sep-Oct;15(5):321-328. PMID: 16958734.
22. Lee HS, Cho YS, Chung WH, Lee SR, Chung J, Hong SH. Obliteration of the temporal dorsal bullae of guinea pigs using hydroxyapatite granules (Bongros®): a radiological and histological study. Korean J Otorhinolaryngol-Head Neck Surg. 2009 1;52(1):14-18.
23. Takahashi S, Nakano Y. A morphological study on obliteration of the temporal dorsal bullae using hydroxyapatite granules. Am J Otol. 1996 3;17(2):197-199. PMID: 8723945.
24. Kveton JF, Friedman CD, Costantino PD. Indications for hydroxyapatite cement reconstruction in lateral skull base surgery. Am J Otol. 1995 7;16(4):465-469. PMID: 8588646.
25. Mahendran S, Yung MW. Mastoid obliteration with hydroxyapatite cement: the Ipswich experience. Otol Neurotol. 2004 1;25(1):19-21. PMID: 14724486.
26. Lee WS, Kim SH, Lee WS, Kim SH, Moon IS, Byeon HK. Canal wall reconstruction and mastoid obliteration in canal wall down tympanomastoidectomized patients. Acta Otolaryngol. 2009 9;129(9):955-961. PMID: 19153845.
27. Park JH, Choi CG, Jeon SR, Rhim SC, Kim CJ, Roh SW. Radiographic analysis of instrumented posterolateral fusion mass using mixture of local autologous bone and b-TCP (PolyBone®) in a lumbar spinal fusion surgery. J Korean Neurosurg Soc. 2011 5;49(5):267-272. PMID: 21716898.
28. Park YH, Kim SG, Lee JW, Yoon YH. Obliteration of temporal dorsal bulla in guinea pigs using different types of calcium phosphate. Int J Pediatr Otorhinolaryngol. 2011 9;75(9):1176-1180. PMID: 21774997.
Fig.┬Ā1Surgical techniques of mastoid obliteration using PolyBone┬«. (A) Inflammatory tissues in the mastoid cavity were completely removed. (B) The mastoid cavity was obliterated with PolyBone┬«. (C) PolyBone┬« in the mastoid cavity was covered with a harvested autologous bone pate. 
Fig.┬Ā3Preoperative and postoperative tympanic membrane (TM) otoscopic findings. (A) Attic destruction was noted in preoperative TM findings (case 1). (B) No retraction in postoperative TM findings (case 1). (C) A retraction pocket was noted in preoperative TM findings (case 4). (D) The retraction pocket had recurred in postoperative TM findings (case 4). 
Fig.┬Ā4Temporal bone computed tomography (CT) before and 12 months after the operation in case 6. The preoperative CT (A, axial; B, coronal) showed mastoid hazziness in the right side. Images obtained at 12 months after surgery (C, axial; D, coronal) demonstrated high density which suggested that the PolyBone┬« graft remained in the right obliterated mastoid area. 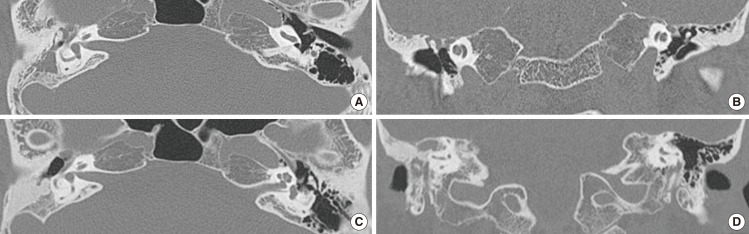
Fig.┬Ā5Complications in case 10. In the intraoperative finding, a wet middle ear mucosa was noted. (A) We blocked the aditus ad antrum with an autologous bone pate, gelfoam and glue. (B) The mastoid cavity was obliterated with PolyBone┬« and covered with a bone pate. (C, D) However, operation site swelling and otorrhea were found on postoperative day 5. We performed a canal wall down mastoidectomy on postoperative day 10. 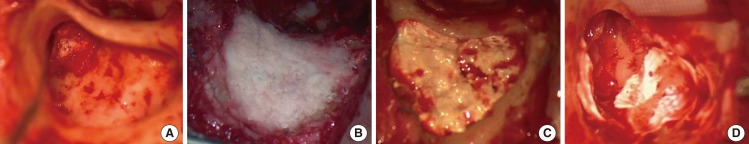
Fig.┬Ā6Computed tomography (CT) images of the skull and bulla at 3, 6, and 9 months after obliteration in the animal study. A-C are coronal views obtained from of the same rat in the skull group, and D-F are from the same rat in the bulla group. After drillings were done in both sides, PolyBone┬« grafts were performed in only the right side. CT images demonstrated high density in only right side, suggesting no bone resorption in the right skull and bulla at 3, 6, and 9 months after obliteration. However, there was no change in the left side skull and bulla (control side). (A, D) Three months after obliteration, (B, E) 6 months after obliteration, (C, F) 9 months after obliteration. 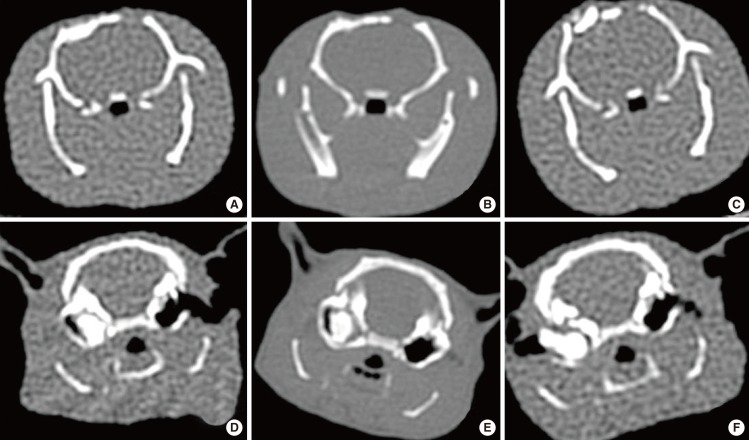
Fig.┬Ā7Histopathologic findings in the skull and bulla at 9 months after obliteration in the animal study. In the control group (A-D), there was no new bone formation. In contrast, active new bone formation was seen without inflammatory infiltration around the implantation field in the PolyBone┬« graft group (E-H), (H&E, A, C, E, and G, ├Ś12.5; B, D, F, and H, ├Ś40). 
|
|
||||||||||||||||||||||||||||||||||||||









