|
 |
- Search
AbstractMethodsA retrospective evaluation of 79 patients who were treated for deep neck infections. The patients were divided into six groups according to weight percentile. Patients who had systemic and/or congenital disease were excluded. Their demographics, etiology, localization, laboratory, and treatment results were reviewed.
ResultsIn total, 79 pediatric patients were recorded: 48.1% were females and 51.9% were males, with a mean age of 7.3 years. In total, 60 patients were under the 50th percentile according to their weight versus all children. The anteroposterior triangle (29.1%) and submandibular (26.5%) spaces were most commonly involved with deep neck infection. However, the anteroposterior triangle space was the highest in the group below the 3rd percentile (44.4%). In the blood analysis, white blood cell levels in patients with at percentile values of 75-50 were higher than other groups (P<0.05). Significant differences were found between C-reactive protein and hemoglobin levels and diameter of abscesses. The need for surgical drainage in patients in lower percentiles was higher. The patients who needed surgical drainage consisted of 56 patients (93.3%) below the 50th percentile and 9 patients (100%) below the 3rd percentile.
Since the discovery of penicillin, the widespread use of antibiotics has reduced the overall incidence of deep neck infections (DNIs) dramatically [1,2,3,4]. While adults often have numerous localizing signs and symptoms, children with DNIs tend to have a more subtle presentation in that they are seldom able to verbalize their symptoms or cooperate with a physical examination [3]. Thus, DNIs continue to occur relatively frequently and can be dangerous in young patients [5]. It is not surprising that the immune system is important in resistance against infection. A healthy diet is necessary for the development of the immune system. For this reason, children in low weight percentiles nearly inevitably show weakness in immune reactions against infection.
We evaluated the effect of weight percentile on DNIs in children. Thus, demography, etiology, location, laboratory findings, and treatment of DNIs in weight percentile groups were examined in this study.
We performed a retrospective analysis of 79 patients, all younger than 18 years old, who were treated in our clinic for DNI. Patients were divided into six groups according to their weight percentile. These groups were defined as 90-75, 75-50, 50-25, 25-10, and 10-3 percentile, and below the 3rd percentile of weight of patients by age [6]. Patients who had systemic and/or congenital diseases (e.g., branchial cleft cysts, thyroglossal duct cysts) were excluded. Patients with Ludwig's angina and dentoalveolar, preauricular, postauricular, external auditory canal, and facial abscesses were also excluded.
The spaces involved were divided according to a description published previously [1] and these are as follows: submandibular, peritonsillar, submental, parapharyngeal, retropharyngeal, anteroposterior triangle, and parotid spaces. Anatomical locations of DNIs were determined using examination findings and radiographic data. Demographic characteristics of the cases, etiological factors, laboratory features, and medical and surgical treatments were analyzed.
In comparisons made according to the weight percentile with these variables, one-way ANOVA was used and Tukey's test was used as a multiple comparison test after ANOVA. Pearson correlation coefficient was calculated to determine linear relationships between the variables.
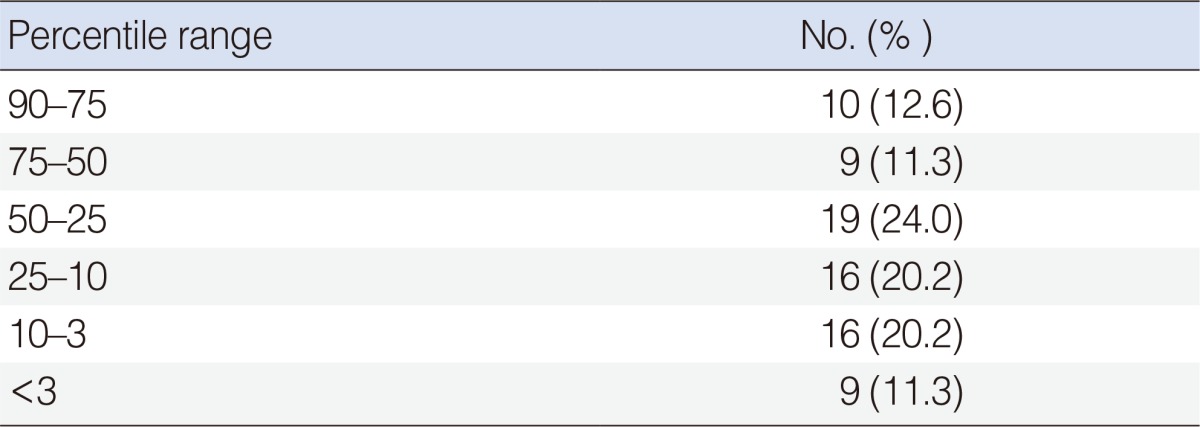
In total, 79 children were treated for DNI. Patients ranged from 2 months to 18 years old, with a mean age of 7.3 years. Of the patients, 38 (48.1%) were females and 41 (51.9%) were males. The percentage distribution of the patients according to weight percentile is shown in Table 1.
The causes of DNI were identified in 50 patients (63.2%). Upper respiratory tract infections were the most common cause of DNIs, usually related to peritonsillar, submandibular, and parapharyngeal space infections. Odontogenic infections were the second most common cause; usually related to submandibular and, parapharyngeal space infections. There was no significant difference in etiology among percentile groups. The etiology of the DNIs is shown in Table 2.
The locations of the DNIs were classified according to the percentile groups based on physical examination and imaging data (Table 3). The anteroposterior triangle space was the most commonly involved space. The anteroposterior triangle space was highest in the group below the 3rd percentile (44.4%).
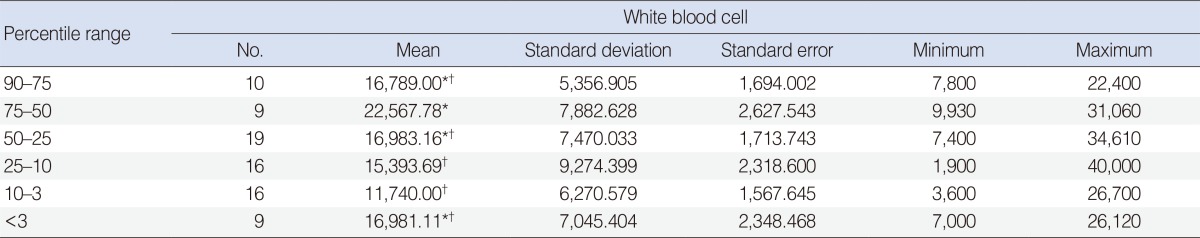
The average white blood cell (WBC) counts in the six groups were determined and compared with each other using one-way ANOVA. Tukey's test was used as a multiple comparison tests after ANOVA. Accordingly, there was a significant difference between patients in the 75-50 percentile and patients in the 25-10 percentile values in WBC counts (P<0.05). WBC counts in patients in the 75-50 percentile group were higher than in the other groups (P<0.05) (Table 4). The statistical relationship between the diameter of abscesses and variations in laboratory parameters is shown in Table 5. A significant negative correlation was found between hemoglobin levels and abscess diameter (P<0.01). A significant positive correlation was found between WBC counts and abscess diameter (P<0.01) and between C-reactive protein (CRP) levels and abscess diameter regardless of disease progression (P<0.05).
Results of pus cultures from surgery or needle aspirations were available for all patients; 27 patients showed bacterial growth. Staphylococcus aureus was the most common bacteria in cultures, followed by Streptococcus pyogenesis. Of the 51 patients where no bacterial growth was observed, 17 had a history of antibiotics usage.
All patients were treated with initial empirical antimicrobial therapy until culture and sensitivity results were available. In total, 60 patients (75.9%) underwent surgical drainages in addition to the medical treatment. The patients who needed surgical drainage consisted of 56 patients (93.3%) below the 50th percentile and 9 patients (100%) below the 3rd percentile. All patients were discharged in stable condition except for one death with mediastinitis that complication of DNIs.
Although antimicrobial therapy has reduced the incidence of deep neck abscesses, these infections remain an important clinical entity, especially in children. The age distribution shows a decreasing incidence with increasing age in children [5]. In our cases, the ratio of female to male cases was about equal, consistent with previous reports [7]. In several pediatric studies of DNIs, the ages of the patients ranged from 3 months to 19 years and the average age was 4.1 years [3,7]. In our study, the ages of the patients ranged from 2 months to 19 years old. Although 45.5% of the patients were under 5 years old, the average age was 7.3, higher than in the literature [7,8,9].
It is important to remember that the development of the immune system is completed with age, based on nutrition [10]. Most of the children in our study were below the 50th weight percentile; indeed, those below the 25th percentile represented more than 50% of the patients. This may explain the difference in average age versus literature reports.
Infections of the upper respiratory tract are the most common causes of neck abscesses in children, because infections of the ears, nose, or throat may spread to the deep neck spaces, by direct continuity or by lymphatic drainage to the lymph nodes [11]. Daya et al. [12] reported the presence of infections of the upper respiratory tract in 48% of 54 patients with deep neck abscesses and Coticchia et al. [7] reported it in 67% in their patients. Similarly, in our study, infections of the upper respiratory tract (30.4%) were the most common cause of DNIs. Odontogenic infections (25.3%) were the second most common cause of DNIs. Although recent trends have shown increases in the prevalence of resistant bacterial strains, DNIs due to pharyngitis or tonsillitis have declined, but DNIs caused by odontogenic infection have increased [1]. However, despite investigations, no specific etiology could be found in 22%-50% of cases [2]. It is possible that the infectious foci had resolved by the time of presentation. In our study, despite all physical examinations, laboratory studies, and imaging methods, no etiological reason was found in 36.7% of our patients. There was no significant difference in etiology among the weight percentile groups.
In recent years, several reports have described the localization and changing trends in deep neck space abscesses [13,14,15]. However, to our knowledge, the location according to weight percentile has not been addressed in DNIs in the literature. Coticchia et al. [7] reported that the distribution of abscesses of the neck was 43% retropharyngeal or parapharyngeal, 32% anterior or posterior triangle, 25% submandibular or submental, and 1% parotid. Similarly Gidley et al. [16] and Ungkanont et al. [17] showed a predominance of retropharyngeal or parapharyngeal abscesses, while Tom and Rice [18] noted the anterior or posterior triangle abscesses to be most common. Beck [19] noted that submandibular or submental abscesses was the most common DNIs. In our study, in contrast to those mentioned above, the most common locations of abscesses in the neck were the submandibular space and the anterior and posterior spaces. The submandibular space was the most common space (45%) in the groups above the 50th percentile groups. On the other hand, the anterior and posterior spaces were the most common spaces (30.5%) in groups below the 50th percentile. However, these differences may have resulted from smaller sample sizes.
Diabetes mellitus, chronic renal failure, hepatic disease, and chronic steroid therapy for autoimmune disease put a patient at increased risk for more severe and atypical infections of the head and neck. Additionally, congenital lesions, such as bronchial cleft cysts, lymphangiomas, thyroglossal duct cysts, and cervical thymic cysts, should be considered in any patient who has recurring DNIs [4,13,17]. Thus, patients with systemic and/or congenital lesions were excluded from this study.
An increase in WBC counts generally reflects resistance by the body, excluding atypical and viral diseases [10]. In our study, a significant difference between patients in the 75-50 percentile group versus patients in the 25-10 percentile group (P<0.05) was observed in WBC counts. Also, WBC counts in patients in the 75-50 percentile group were higher than in the other groups (P<0.05). We also observed a significant negative correlation between hemoglobin levels and abscess diameter. According to some authors, B lymphocyte function decreases with iron deficiency and humoral immunity is attenuated [10,20]. This may explain why the patients with low weight percentile values were prone to complications and needed surgical drainage more commonly.
Cultures from aspirates from deep neck abscesses are commonly polymicrobial and reflect the oropharyngeal flora and their odontogenic nature [21]. The organisms are typically both aerobic-such as Streptococcus viridans, beta-hemolytic streptococcus, S. aureus, and Klebsiella pneumoniae-and anaerobic, such as Bacteroides and Peptostreptococcus [1,2]. However, Coticchia et al. [7] reported that the organisms isolated from anterior or posterior triangle abscesses (35%) and submandibular abscess (42%) were mostly S. aureus. Similarly, Dodds and Maniglia [22] noted that the most commonly isolated organism was S. aureus in their study. In our study, of the pus cultures, 34.2% were positive. S. aureus was the most commonly isolated organism (46.4%), followed by S. pyogenesis. Additionally, the organisms isolated were similar across the different weight percentile groups. Thus, our results were largely consistent with those of Coticchia et al. [7] and Dodds and Maniglia [22].
All patients with suspected DNIs underwent ultrasonography to identify the extent of the infections and to differentiate cellulitis from abscesses. Magnetic resonance imaging (MRI) and contrast-enhanced computed tomography (CT) are helpful in deciding whether surgical intervention is indicated [23]. However, CT does not always accurately differentiate abscesses from cellulitis [24]. In our study, contrast-enhanced CT was used to detect the extent of the abscess in some patients.
All patient with a DNI should be given initial empirical antibiotic therapy until culture and sensitivity results are available. Empirical therapy should be effective against the aerobic and anaerobic bacteria that are commonly involved, and once available, the results of the culture and sensitivity tests can allow for tailoring of appropriate antibiotic therapy. Penicillin in combination with a ╬▓-lactamase inhibitor (e.g., amoxicillin or ticarcillin with clavulanic acid) or a ╬▓-lactamase-resistant antibiotic (e.g., cefoxitin, cefuroxime, imipenem, meropenem) in combination with a drug that is effective against most anaerobic bacteria (e.g., clindamycin, metronidazole) is recommended for optimal empirical coverage [17]. In our study, initial empirical antimicrobial therapy was given to all patients until culture and sensitivity results were available. Generally, regimens included the ceftriaxone plus metronidazole combination. Of the 79 patients who had not previously taken an antibiotic, 19 (24.1%) responded effectively to only intravenous antimicrobial therapy. Several studies have shown that previous antibiotic use was associated with increased antimicrobial resistance [19].
Surgical drainage is the classical approach to any DNI with suspected abscess formation and is still considered by many investigators to be the first-line treatment in such cases [1,7,13,24]. Indications for surgery include airway compromise, critical conditions, septicemia, complications, descending infection, diabetes mellitus, or no clinical improvement within 48 hours of the initiation of parenteral antibiotics. Additionally, abscesses greater than 3 cm in diameter that involve the prevertebral, anterior visceral, or carotid spaces, or that involve more than two spaces, should be drained surgically [1]. In our study, surgical drainage was needed in 60 of 79 patients (75.9%); higher than in literature reports [4,7,12,25]. This may have been because most of our patients were below the 50th weight percentile, and the need for surgical drainage was higher in patients at lower percentiles. For example, an indication for drainage in patients above the 50th percentile was seen in 31% (6/19), whereas this ratio in those below the 10th percentile was 80% (20/25).
In conclusion, the anteroposterior space is most commonly involved in DNIs in children below the 50th weight percentile. The course of DNIs was worse in patients with low WBC counts, high CRP levels, low hemoglobin levels, and low weight percentiles, especially those below the 3rd percentile. Thus, special attention should be paid to the risk of complications in these patients.
ACKNOWLEDGMENTSWe thank the Biostatistics Unit (Chief director, Siddik Keskin, PhD) for help with the statistical analyses and interpretation of the data.
References1. Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. 2008 6;41(3):459-483. PMID: 18435993.
2. Weed HG, Forest LA. Deep neck infection. In: Cummings C, Flint P, Harker L, Richardson M, Robbins T, Schuller D, editors. Otolaryngology head and neck surgery. Philadelphia, PA: Mosby; 2005. p. 2515-2524.
3. Barratt GE, Koopmann CF Jr, Coulthard SW. Retropharyngeal abscess: a ten-year experience. Laryngoscope. 1984 4;94(4):455-463. PMID: 6708689.
4. Choi SS, Vezina LG, Grundfast KM. Relative incidence and alternative approaches for surgical drainage of different types of deep neck abscesses in children. Arch Otolaryngol Head Neck Surg. 1997 12;123(12):1271-1275. PMID: 9413352.
5. Thompson JW, Cohen SR, Reddix P. Retropharyngeal abscess in children: a retrospective and historical analysis. Laryngoscope. 1988 6;98(6 Pt 1):589-592. PMID: 3374231.
6. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 6;(314):1-27. PMID: 11183293.
7. Coticchia JM, Getnick GS, Yun RD, Arnold JE. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg. 2004 2;130(2):201-207. PMID: 14967751.
8. Cabrera CE, Deutsch ES, Eppes S, Lawless S, Cook S, O'Reilly RC, et al. Increased incidence of head and neck abscesses in children. Otolaryngol Head Neck Surg. 2007 2;136(2):176-181. PMID: 17275535.
9. Nagy M, Pizzuto M, Backstrom J, Brodsky L. Deep neck infections in children: a new approach to diagnosis and treatment. Laryngoscope. 1997 12;107(12 Pt 1):1627-1634. PMID: 9396677.
10. English BK, Watson CB. The neonatal immune system in clinical immunology. In: Rich RR, Fleisher TA, Schwartz BD, Shearer WT, Strober W, editors. Clinical immunology: principles and practice. St. Louis, MO: Mosby; 1996. p. 779-788.
11. Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004 12;62(12):1545-1550. PMID: 15573356.
12. Daya H, Lo S, Papsin BC, Zachariasova A, Murray H, Pirie J, et al. Retropharyngeal and parapharyngeal infections in children: the Toronto experience. Int J Pediatr Otorhinolaryngol. 2005 1;69(1):81-86. PMID: 15627452.
13. Huang TT, Liu TC, Chen PR, Tseng FY, Yeh TH, Chen YS. Deep neck infection: analysis of 185 cases. Head Neck. 2004 10;26(10):854-860. PMID: 15390207.
14. Sethi DS, Stanley RE. Deep neck abscesses: changing trends. J Laryngol Otol. 1994 2;108(2):138-143. PMID: 8163915.
15. Cmejrek RC, Coticchia JM, Arnold JE. Presentation, diagnosis, and management of deep-neck abscesses in infants. Arch Otolaryngol Head Neck Surg. 2002 12;128(12):1361-1364. PMID: 12479720.
16. Gidley PW, Ghorayeb BY, Stiernberg CM. Contemporary management of deep neck space infections. Otolaryngol Head Neck Surg. 1997 1;116(1):16-22. PMID: 9018251.
17. Ungkanont K, Yellon RF, Weissman JL, Casselbrant ML, Gonzalez-Valdepena H, Bluestone CD. Head and neck space infections in infants and children. Otolaryngol Head Neck Surg. 1995 3;112(3):375-382. PMID: 7870436.
18. Tom MB, Rice DH. Presentation and management of neck abscess: a retrospective analysis. Laryngoscope. 1988 8;98(8 Pt 1):877-880. PMID: 3398666.
19. Beck AL. The influence of the chemotherapeutic and antibiotic drugs on the incidence and course of deep neck infections. Ann Otol Rhinol Laryngol. 1952 6;61(2):515-532. PMID: 14944151.
20. Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997 8;66(2):464S-477S. PMID: 9250134.
21. Rega AJ, Aziz SR, Ziccardi VB. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J Oral Maxillofac Surg. 2006 9;64(9):1377-1380. PMID: 16916672.
22. Dodds B, Maniglia AJ. Peritonsillar and neck abscesses in the pediatric age group. Laryngoscope. 1988 9;98(9):956-959. PMID: 3166090.
23. Miller WD, Furst IM, Sandor GK, Keller MA. A prospective, blinded comparison of clinical examination and computed tomography in deep neck infections. Laryngoscope. 1999 11;109(11):1873-1879. PMID: 10569425.
24. Ridder GJ, Technau-Ihling K, Sander A, Boedeker CC. Spectrum and management of deep neck space infections: an 8-year experience of 234 cases. Otolaryngol Head Neck Surg. 2005 11;133(5):709-714. PMID: 16274797.
25. Oh JH, Kim Y, Kim CH. Parapharyngeal abscess: comprehensive management protocol. ORL J Otorhinolaryngol Relat Spec. 2007;69(1):37-42. PMID: 17085951.
|
|
||||||||||||||||||||||||||||||||||||||||||