INTRODUCTION
Esophagectomy and esophagogastrostomy is a standard procedure in surgical treatment of esophageal cancer. However, leakage of anastomosis site results in critical morbidity and mortality. In management of this leakage problem, endoscopic intervention can take a role during conservative approach, and can be an alternative of surgical approach. Esophageal stents are placed to treat leakage problem with satisfying result in experienced centers [1]. However, there has not been a documented case in Korea. We present a case in which anastomosis leakage was successfully controlled by stent insertion.
CASE REPORT
The patient was 65-year-old gentleman who had history of old medullary infarction without sequele. With social history of heavy drinking and smoking of 20 pack-year, he visited out-patient clinic due to dysphagia and chest pain. On esophagogastroduodenoscopy, ulcerofungating mass on esophageal mucosa at 30–33 cm below upper incisor was detected and biopsied. The pathologic report of the mass was invasive squamous cell carcinoma. Muscle layer invasion was suspicious in endoscopic ultrasound finding. There was no evidence of systemic or regional lymph node metastasis.
Robot-assisted Ivor Lewis operation was performed. Both abdominal and thoracic procedures were performed by robot-assisted technique. Gastric conduit was made intracorporeally and intra-abdominal lymph nodes were dissected to the level of celiac axis. Pyloromyotomy was performed and jejunostomy was made at proximal jejunum. In thoracic phase thoracoscopic adhesiolysis was performed due to severe pleural adhesion. After esophagectomy, mediastinal lymph node dissection up to both recurrent laryngeal lymph nodes was performed. With 25-mm-sized endoscopic end-to-end anastomosis stapler, intrathoracic esophagogastrostomy was carried out above azygos vein. Reinforcement sutures with 4-0 black silk were placed on the anastomosis site.
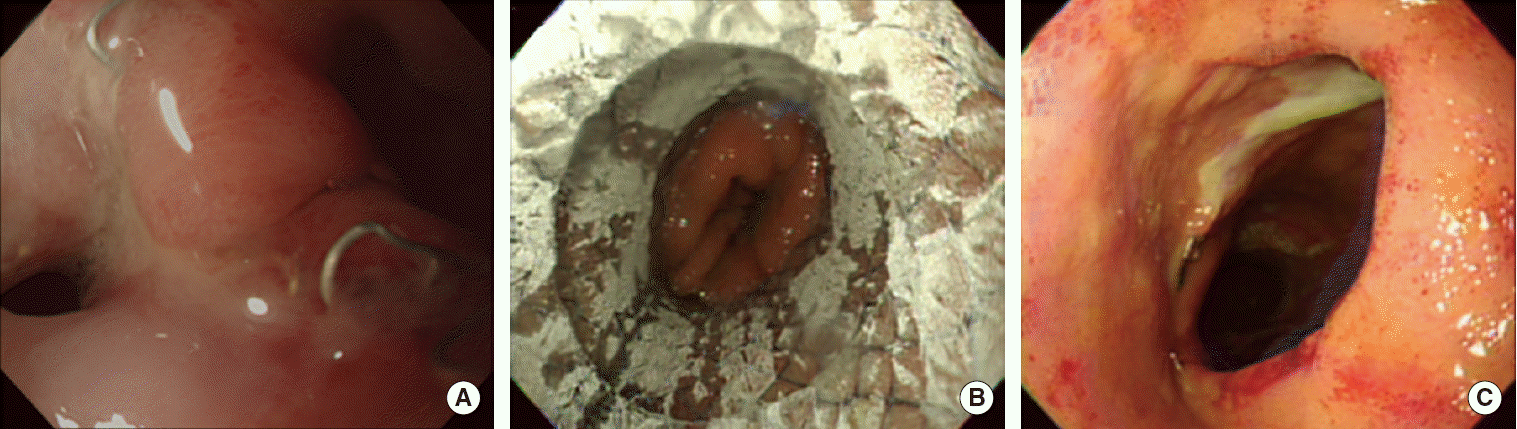
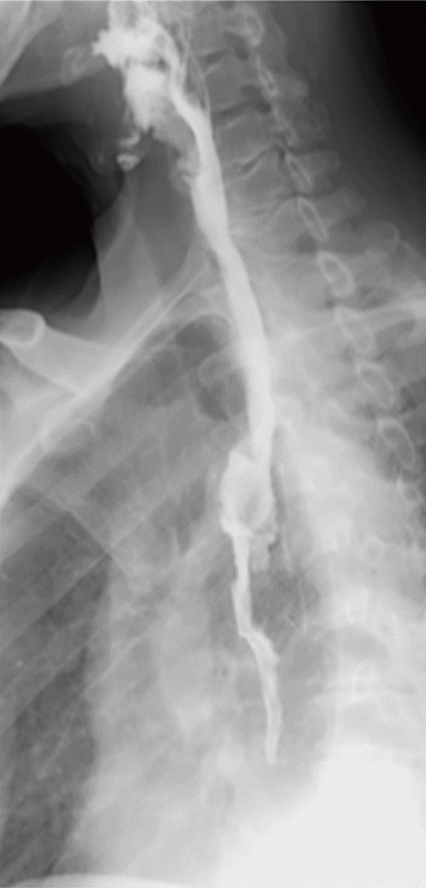
On the third postoperative day, high fever up to 38.4°C occurred and blood exam demonstrated leukocytosis and elevated C-reactive protein. At the following day, color of chest tube drainage changed to dark greenish and total bilirubin concentration from chest tube drain was 6.5 mg/dL. Chest computed tomography revealed loculated pleural effusion on right thoracic cavity. For drainage of effusion and identification of leakage point, thoracoscopic exploration was performed. However, gross leakage point could not be identified after draining loculated contaminated fluid. Fever was controlled and general condition of the patient was improved rapidly. However persistent leukocytosis was not normalized 2 weeks after thoracoscopic exploration. Esophagoscopy was performed 17 days after thoracoscopic exploration to detect any residual leakage point. Esophagoscopy demonstrated multiple ulcer and a leakage hole on anastomosis site (Fig. 1A). Covered metal stent (18 mm × 60 mm; Bonastent Esophageal, EndoChoice Inc., Alpharetta, GA, USA) was placed in leakage site (Fig. 1B). Two weeks after stent insertion, esophagoscopy was performed to check proper position of stent, 2 weeks thereafter, the stent was removed endocopically (Figs. 1C, 2). No evidence of leakage was found in esophagogram. One week after stent removal, patient was discharged with full nutritional intake via oral route exclusively. The patient is now on regular outpatient follow-up without evidence of anastomosis site stricture for 10 months after esophagectomy.
DISCUSSION
The incidence of anastomotic leakage after esophagectomy is estimated 11% by a recent review of The Society of Thoracic Surgeons general thoracic surgery database. And the influence of this significant complication pose the patient in high mortality of 35.7% contrast to 4.2% of those without leakage [2]. As conventional treatment, surgical repair of the anastomosis site leakage demonstrated 71.4%–100% success rate.
In 1996, Segalin et al. [3] reported 2 successful cases of endoscopic esophageal stent placement for the treatment of postoperative esophageal leakage. Prior to the report, endoluminal prosthesis had been adopted to treat malignant fistula caused by esophageal cancer, but not in postoperative setting. Reports on esophageal stent for the treatment of postoperative leakage arose around 2005. The success rates of esophageal stent range from 59.1% to 94.1%. Of those successful cases, included were an anastomotic leak encompassing approximately 50% of the original anastomotic circumference and a leakage whose size was larger than 5 cm. Although stent migration was the most common complication, endoscopic removal and reinsertion of stent was feasible. Other than stent migration, stricture after stent removal and tracheoesophageal fistula were reported as related complication, and one patient expired due to fatal gastro-aortic fistula [4-6]. In 2011, Freeman et al. [2] reported 94.1% success rate in acute intrathoracic anastomotic leakage after esophagectomy. When it comes to the excellent occlusion rate of theirs, exclucion criteria should be remarked such as leakage from gastric conduit itself remote from the anastomosis, complete dehiscence of the anastomosis and conduit other than stomach.
While the number of robot-assisted esophagectomy has been increasing, anastomotic leakage is still one of the common complications. As in our case, stent insertion can be helpful in the aspect of noninvasiveness, which was minimized by robot-assisted procedure.
There has not been yet any prospective clinical study, which compared the outcome of esophageal stent to that of conservative/surgical treatment. Only a retrospective study comparing esophageal stent with conventional treatment was published and revealed esophageal-stent-favoring outcome with better leakage control, shorter length of hospital stay [7]. Esophageal stent might not be successful in some cases if the defect is large, or if the viability of tissue around defect is low, which is the common situation of patients with neoadjuvant chemoradiation treatment for locally advanced esophageal cancer. However in the series which Freeman et al. reported, 15 patients with neoadjuvant chemoradiation were included in successful cases [2]. It implicates that further application and spread of indication are forecasted. Not only substituted for the surgical repair, esophageal stents can also take a supplementary role to the surgical repair, functioning as buttress and barrier to gastrointestinal contents [8]. Still there are a lot of questions to be answered, such as the outcomes in patient with trachea-esophageal fistula or with leakage at the site of conduit necrosis, etc. Answering the remained questions, further utilizing of the device is being anticipated.