|
 |
- Search
AbstractObjectivesDespite the ongoing development of treatment protocols for laryngeal squamous cell carcinoma (LSCC), the patients suffering with this malady have shown only a modestly improved outcome. This poor outcome has been attributed to the lack of therapy that's individualized to the tumor's biological properties. Various studies have showed that galectin-8 is widely expressed in tumor tissues as well as in normal tissues, and the level of the galectin-8 expression may correlate with the malignancy of human squamous cell carcinoma. The purpose of this study is to evaluate the expression of galectin-8 and to investigate its correlations with the primary stage, the nodal involvement, the clinical stage and the histologic grade of squamous cell carcinoma of the larynx.
MethodsThe paraffin-embedded tissue specimens from 77 patients who were diagnosed as LSCC between 1993 and 2007 were immunohistochemically stained for galectin-8.
The malignant tumors in the upper respiratory tract and the upper digestive organs have some unique structural, pathological and dynamic characteristics in terms of their developmental process. Head and neck cancers are mostly squamous cell carcinomas and they are divided into well-differentiated, moderately-differentiated and poorly-differentiated lesions (1). Squamous cell carcinoma of the larynx has high morbidity and mortality rates. Its treatment has recently become somewhat better as the medical treatments have improved and new therapies have been developed, but the index used to determine the proper therapeutic methods and to judge the prognosis of this malady is still not sufficient. Thus, various studies are currently being conducted to determine the malignancy of this type of tumor. If researchers can find a factor to objectively appraise the biological characteristics of a cancer from the samples of tumor tissues, for example, appraising the malignancy of the cancer cells, then this will be a more releiable prognostic factor than the clinical stage, which is the factor that has been used for this in the past (2).
Galectin-8 is one of the galectins, which are a group of mammal lectins. It is a 34 kDa protein and it is composed of two carbohydrate-recognition domains (CRDs). It gets coupled by different lengths of "link peptide" (3, 4). The same as other galectins, it is secreted as a secretory protein to become a matrix protein that gets connected with some selective parts of the integrins on the surface of a cell or it becomes a matrix protein that forms a group of proteins that play a role for encouraging cell adhesion in a fashion similar to fibronectin (5). The existing studies have shown that galectin-8 has a positive relation with some human cancers, and especially with prostatic carcinoma (6).
Recently, the usefulness of galectin-8 has been researched, but its roles in the head and neck cancers has not been studied yet. Therefore, this paper intends to examine the correlations between the expression pattern of galectin-8 in squamous cell carcinoma of larynx and T-stage of the tumor, metastasis to the lymph node, clinical stage, and histopathologic differentiation. And based on the examination, it will study the possibility of galectin-8 as a prognostic factor. For this study, immunohistochemical staining will be peformed on galectin-8.
Seventy-seven cases of squamous cell carcinoma of larynx that were clearly diagnosed via biopsy from 1993 to 2007 were used for this study. They were classified by the differentiation of the tumor and the stage of the disease. The patients were 29-81 yr old (average age: 63); 72 of them were males (93.5%) and 5 were females (6.5%). In terms of the histopathologic differentiation, 23 cases were well-differentiated, 17 cases were moderately-differentiated, and 5 were poorly-differentiated. It was impossible to confirm the histologic differentiation of 32 cases, and these were excluded from the statistical analysis of histologic differentiation. The T-stage and the clinical stage, according to the presence of cervical lymph node metastasis, were classified by the American Joint Committee on Cancer (AJC) classification (2002). Based on the T-stage, 16 cases were T1, 20 cases were T2, 26 cases were T3, and 15 cases were T4. Based on the N-stage, 55 cases were N0, 10 cases were N1, 10 cases were N2, and 2 cases were N3. Based on the clinical stage, 14 cases were 1st stage, 16 cases were 2nd stage, 23 cases were 3rd stage, and 24 cases were 4th stage. The biopsy report and medical treatment records of each patient were reviewed to determine the TNM stage and the histologic differentiation.
Galectin-8 (H-80, SC-28254) manufactured by Santa Cruz Biotechnology (Santa Cruz, CA, USA) was used as the primary antibody for the immunohistochemical tests, and a Labeled Streptavidin Biotin (LSAB) kit and diaminobenzoic acid (DAB) kit made by Dako (Glostrup, Denmark) were used as the secondary antibody and the color former, respectively. Counterstaining was performed with Mayer's hematoxylin and a universal mount made by Research Genetics (Huntsville, AL, USA) was used for sealing.
The lesions of the larynx were re-examined by using the slide files of the tissues at the laboratory, and the presence of cancer infiltration was re-checked. The representative parts suitable for the purposes of this research were chosen and then slides were made for the immunohistochemical tests.
Paraffin embedded tissues that had been fixed in 10% neutral buffered formalin were cut to 4 ┬Ąm thickness and then they were attached to X-traŌäó slides (Surgipath, Richmond, IL, USA). They were deparaffined by using xylene and then they were treated with 90% non-aqueous alcohol and 75% and 50% ethanol for 2 min, respectively. They were then stained by the LSAB method. For the recovery of antigen, the slides were put into citrate buffered solution (10 mM, pH 6.0) and this was boiled in a microwave for 15 min. They were left at room temperature for 20 min and next washed with 50 mM Tris buffered solution (TBS, pH 7.5). In order to suppress the activation of intrinsic peroxidase in the tissue sections, the slides were treated with 0.3% hydrogen peroxide-methanol for 10 min and then they were washed with distilled water. They were reacted with a growth blocking antibody at room temperature for 10 min and then they were reacted with the primary antibody galectin-8 (dilution-1:400) for 1 hr. They were washed with Tris buffered solution and then reacted with the secondary antibody containing biotin at room temperature for 10 min with using LSAB kits. After this they were washed with tryptic soy broth (TSB) and made to react with peroxidase-coupled streptavidin solution for 10 min. They were washed again with Tris buffered solution and then reacted with diaminobenzidine (DAB). Counterstaining was performed with Mayer's hematoxylin and universal mount was used for sealing. As a negative control group, Tris buffered solution was used instead of primary antibody.
The results of the immunohistochemical stainings were read by a pathologist who was without knowledge of the clinical information. The cases for which galectin-8 was stained on the cytoplasm of a cancer cell in the form of brown granules were judged positive and we compared the percentages of the positively stained cancer cells out of more than 1,000 cancer cells within the tumor region. The case that the cancer cells were stained less than 1% in 100 magnified fields was scored at 0. The case that cancer cells were stained less than 10% was scored as 1 point, the case that the cancer cells were stained 11-50% was scored as 2 points and 3 points were given when more than 51% of the cancer cells were stained.
Depending on the strength of staining, the samples were classified into negative (0 point), weak positive (1 point), moderate positive (2 points) and strong positive (3 points). If the points for the strength of staining and the points for the distribution were added up as 0-2 points, then this was classified as category I. If they are added up as 3-4 points, then this was classified as category II and if they are added up as 5-6 points, then this was classified as category III. Category I was judged as negative while categories II and III were judged as positive (7).
Correlations between the relative extent of galectin-8 in the lesions of squamous cell carcinoma of the larynx and the prognostic factors (the size of the original cancer, metastasis to the cervical lymph node, the clinical stage and the histopathological differentiation) were tested by use of Chi-square tests. P<0.05 was regarded statistically significant.
A total of 77 tissue samples of squamous cell carcinoma of the larynx were classified by clinical stage: 30 cases were early cancer (Stages I and II) and 47 cases were advanced cancer (Stages III and IV). Based on the T-stage, 37 cases were classified as early stage (T-stages 1 and 2) and 41 cases were advanced stage (T-stages 3 and 4). Twenty-two cases were classified as a group showing metastasis to the cervical lymph nodes (N Ōēź 1) and 55 cases were classified as the group showing no metastasis to the cervical lymph nodes (N0). Depending on the histologic differentiation, the cases of squamous cell carcinoma were classified as 23 cases of well-differentiated tumor, 17 cases of moderately-differentiated tumor and 5 cases of poorly-differentiated tumor. 32 cases couldn't be classified (Table 1).
Among the 77 samples, there was no case (0%) showing that cancer cells were stained less than 1% at 100 magnifications. There was 1 case (1.3%) showing that cancer cells were stained less than 10%. 18 cases (23.4%) showed 11-50% staining while 58 cases (75.3%) showed more than 51% staining. Based on the strength of the staining, 0 cases (0%) were negative, 11 cases (14.2%) were weakly positive, 30 cases (39.0%) were moderately positive and 33 cases (42.8%) were strongly positive. Category I, whose total points for the strength of staining and the points for distribution were 0-2, had 1 case (1.3%). Category II (3-4 points) had 21 cases (27.3%) and category III (5-6 points) had 55 cases (71.4%) (Table 2).
Immunohistochemical staining showed a strong reaction around the part of the squamous cell carcinoma of the larynx, and it was especially observed to be stronger on the part where the cancer cells invaded the stroma (Fig. 1). Compared with the normal tissues or the squamous epithelium with dysplasia, the expression of galectin-8 was stronger around the cancer cells (Fig. 2).
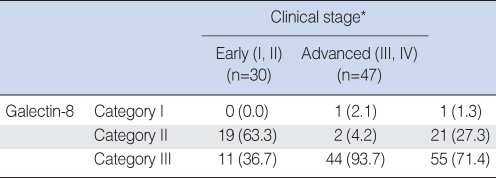
For the study of the positive expression of galectin-8 based on the T-stage, T1 and T2 were classified as the early group (n=36), and T3 and T4 were classified as the advanced group (n=41). For the early stage group, category I had no cases, category II had 19 cases and category III had 17 cases. In the advanced group, category I had 1 case, category II had 2 cases and category III had 38 cases, and the differences between the groups were statistically significant (P<0.05) (Table 3).
Based on the clinical data, the cases were divided into the group that had no metastasis to the cervical lymph nodes (N0) (n=55) and the group that had metastasis to the cervical lymph nodes (NŌēź1) (n=22). In the group that had no metastasis to the cervical lymph nodes (N0), category I had 1 case, category II had 21 cases and category III had 33 cases. In the group that had metastasis to the cervical lymph nodes (NŌēź1), category I had 0 case, category II had 0 case and category III had 22 cases, and the differences between the groups were statistically significant (P<0.05) (Table 4).
The cases were divided into two groups: the group with early stages 1 and 2 (n=30) and the group with advanced stages 3 and 4 (n=47). In the early stage group, category I had 0 case, category II had 19 cases and category III had 11 cases. In the advanced stage group, category I had 1 case, category II had 2 cases and category III had 44 cases, and the differences between the groups were statistically significant (P<0.05) (Table 5).
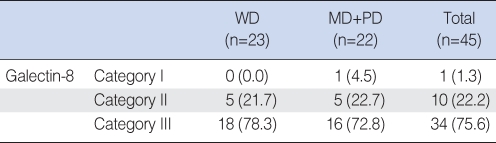
The cases were divided into three groups: well-differentiated (n=23), moderately-differentiated and poorly-differentiated (n=22). In the well-differentiated group, category I had 0 case, category II had 5 cases and category III had 18 cases. In the moderately and poorly-differentiated group, category I had 1 case, category II had 5 cases and category III had 16 cases. Category III had more cases, but this was not statistically significant (Table 6).
Squamous cell carcinoma of the larynx can be completely cured by operations, but it can inevitably cause complications, including dysphonia. In spite of the evolution of operative techniques, radiotherapy and anticancer medicine, the success rate for treating squamous cell carcinoma of the larynx and the patient survival have not been greatly improved. Under these circumstances, it is urgent to develop new treatment methods. To do this, it is important to make treatment plans at the early stage of the cancer and to use a prognostic factor with which a doctor can diagnose and cure the cancer early (8).
The prognosis of squamous cell carcinoma of the larynx is determined by several factors (factors of the cancer, factors of a patient and factors of treatment) (2). Among those prognostic factors, the clinical stage (a factor of the cancer) has been used most often, but the clinical progress and the prognosis can be different depending on tumor's origin, the biological characteristics of the cancer cell, and the differences in the cellular/microscopic environment, even though the clinical stage is same. Therefore, studies have been conducted to determine the prognosis by measuring the malignancy of the cancer cells (9).
Along with the rapid development of molecular biology, cell biomarkers have been used as a variable for appraising the clinical malignancy of a cancer (10). Studies on the expression of cancer protein by performing immunohistochemical staining are being actively conducted, but the research on galectin-8 is not sufficient and any studies on the head and neck cancers are especially rare.
Galectin is 31 kDa carbohydrate-coupled protein that belongs to the beta-galactoside-binding animal protein lectin family. The chief characteristic of this protein family is that it shares carbohydrate recognition binding domains (CRDs). Depending on the number and the structure of the CRDs, it is classified into the proto type, the tandem repeat type and the chimera type (11, 12). At present, a total of 14 kinds of galectin protein have been found. The proto type has one CRD and galectin -1, -2, -5, -7, -10, and -13 are the proto type. The tandem repeat type has two CRDs, which includes galectin -4, -6, -8, -9, -12, and -14, and the CRDS are connected by a hinge peptide. Galectin-3 has one CRD, which contains a Pro-Gly-rich repeat unit, so it belongs to the chimera type (13, 14). It is known that galectins are in epithelial cells and immune cells and they are involved in such biological functions as embryogenesis, cell growth, cell adhesion, cell proliferation, apoptosis and mRNA splicing (15-17). In addition, this protein is known to be distributed in the cytoplasm, nuclei, cell walls and extracellular matrix, and this protein is related with tumorigenesis and metastasis (18). The human galectin-8 gene (LGALS8) conforms to 33 kbp of DNA. Galectin-8 is located at chromosome 1 (1q42.11), which includes 11 exons. It encodes mRNA through splicing of 14 different transcripts (6). This mRNA encodes 6 isoform proteins of galectin-8 and among them, 3 belong to the tandem repeat galectin type, while another 3 belong to the proto type. The proto type galectin-8 has never been isolated and it has been hypothetically deducted from the separated transcripts (19). Therefore, galectin-8 belongs to the subtype of the tandem repeat type galectin. It includes 2 CRDs and they are connected by a 32-amino acid linker (Fig. 3) (6). At the level of hexane, galectin-8 is consistent with galectin-4 with 50% homology, and at the level of amino acids, it shows 34% homology (3, 20). However, galectin-4 and galectin-8 play similar or different roles in the generation of cancer is not known (21). The previous studies have reported that galectin-8 is a controller of stromal cells as is related with cell adhesion, and its function requires that both CRDs should be activated (5, 22). This has been revealed to be different in normal cells and cancer cells, and so galectin-8 is helpful for understanding the growth and differentiation of malignant tumors (23-26).
Galectin-8 is extensively expressed in normal tissues (brain, breast, large intestine, retina, kidney, pancreas, placenta, spleen, testicle, uterus, blood vessel, esophagus and heart), and it is also distributed in cancer tissues (brain, breast, large intestine, germ cells, head and neck, kidney, muscles, ovary, pancreas, thyroid gland, placenta, prostate, uterus, lung, stomach and esophagus cancer) (3, 27-30). Danguy et al. (24) compared the expression of galectin-8 by the type of an organ according to immunohistochemical staining on the cancer tissues that correspond with normal tissues. They stated that for the tissues of the large intestine, the pancreas, the liver, the skin and the larynx, the cancer tissues had a lower expression of galectin-8 than did the normal tissues; for the tissues of the breast, there was no difference between the cancer tissues and the normal tissues, and the expression of galectin-8 was increased in the cancer tissues of the lungs, the bladder, the kidney, the prostate and the stomach. Thus, galectin-8 expression for the malignant transformation of an epithelial originated tissue, immunohistochemical monitoring of galectin-8 reveals an organ-type-dependent regulation. There is a report that the galatin-8 expression is reduced in the laryngeal squamous cells as compared with that of normal tissues.
In our study, we didn't compare the extent of the galectin-8 expression between the normal larynx tissues and the tissues of squamous cell carcinoma of the larynx, but we found an increased expression of galectin-8 that was caused by an increase in the malignancy of the squamous cell carcinoma of the larynx. A study on the expression of galaction-8 in normal tissue as compared with that in the corresponding cancer tissues needs to be conducted in the future.
Su et al. (31) have shown that the secretion and the surface expression of galectin-8 were very high in invasive prostatic carcinoma, early prostatic carcinoma and prostatic carcinoma in situ, but galactin-8 was not increased in the normal prostatic tissues and the tissues of benign prostatic hypertrophy. Because of this reason, galectin-8 was called Prostate Cancer Tumor Antigen-1 (PTCA-1) in the past (31). The excessive expression of galectin-8 was observed in the cells of lung cancer, the cancer cells of the central nervous system (astrocytoma and glioblastoma) and the cancer cells of skin T-cell lymphoma and cholesteatoma (26, 32, 33). This excessive expression of galectin-8 seems to be a key characteristic that is connected with the progress of some tumors.
Nagy et al. (25) studied the expression of galectin-8 in colon cancer and they found that depending on the change in the malignancy of the colon tissues, the immunohistochemical expression of galectin-8 was remarkably decreased, and as the malignancy of a tumor became higher, the amount of galectin-8 protein was reduced so that the expression of galectin-8 was lower in the cancer tissues than that the normal tissues or the benign tissues of the colon cancer. This result is completely opposite to the result of this research that the expression of galectin-8 was increased as the malignancy of the squamous cell carcinoma of larynx increased. So, this suggests that the expression of galectin-8 caused by malignancy is different according to the kind of an organ.
An immunohistochemical staining study was previously conducted on the primary and secondary lung cancers of various histologic types (29, 30). Henno et al. (29) found that galectin-8 was strongly expressed in squamous cell carcinoma of the lung, but it was weakly expressed in adenocarcinoma and it was not revealed at all in small-cell carcinoma. By observing the relations between the expression of galectin-8 and the differentiation of squamous cell carcinoma and neuroendocrine tumor, they insisted that the expression of galectin-8 was related with the type of a tumor. Through those researches, it will be possible to clarify the same relation (the expression of galectin-8 increases with the increased malignancy of squamous cell carcinoma of the larynx) in other tumors. In addition, it might be possible to use galactin-8 as a prognostic factor for various tumors, and especially squamous cell carcinoma.
The difference in the expression of galectin-8 between normal tissues and tumor tissues has recently been used as a guide for the treatment and for selecting a preventive method for treating squamous cell carcinoma of the lungs (19). These various experiments are now being reviewed and appraised, but they show the different potentialities of galectin-8 and further, they help us increase our understanding of this protein. In addition, these experiments show the possibility that galactin-8 can be used to block tumorigenesis.
We performed immunohistochemical staining for galctin-8 on 77 cases of squamous cell carcinoma of the larynx, and the correlations between the expression of galectin-8 and the tumor stage, metastasis to the lymph nodes, the clinical stage and the histologic differentiation were examined and statistically analyzed. The following conclusions were made.
Galectin-8 seems to be closely related to the tumorigenesis of squamous cell carcinoma of the larynx and its malignancy, while it also controls cell adhesion and the interactions between cells and the stroma of the larynx during various physiological and pathological progresses. The expression of galectin-8, depending on the tumor stage, metastasis to the lymph nodes and then clinical stage, was statistically significant enough to be used as a clinical prognostic factor for squamous cell carcinoma of the larynx. We found that the expression of galactin-8 was not statistically related with histologic differentiation. However, more cases are needed to confirm this and a study that focuses on comparing the expression of galactin-8 between normal tissues and cancer tissues needs to be done in the future to further determine the correlation of galaction-8 with other factors.
References1. Schantz SP, Harrison LB, Forastiere AA. In: DeVita V, Hellman S, Rosenberg S, editors. Tumors of the nasal cavity and paranasal sinuses, nasopharynx, oral cavity, and oropharynx. Cancer: principles and practice of oncology. 1997. 5th ed. Philadelphia (PA): JB Lippincott; p. 741-847.
2. Park IS, Kim SH, Jung YG, Rho YS, Kim HJ, Lim HJ. Clinical significance of the expression of TGF-a and TGF-╬▓ in squamous cell carcinoma of the larynx and hypopharynx. Korean J Otolaryngol-Head Neck Surg. 1996 6;39(6):980-989.
3. Hadari YR, Paz K, Dekel R, Mestrovic T, Accili D, Zick Y. Galectin-8: a new rat lectin, related to galectin-4. J Biol Chem. 1995 2 17; 270(7):3447-3453. PMID: 7852431.
4. Hadari YR, Eisenstein M, Zakut R, Zick Y. Galectin-8: on the road from structure to function. Trends Glycosci Glycotechnol. 1997 1;9(45):103-112.
5. Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, et al. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci. 2000 7;113(Pt 13):2385-2397. PMID: 10852818.
6. Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J. 2004;19(7-9):517-526. PMID: 14758075.
7. Shin DH, Lee CH, Kang HJ, Sol MY, Lee MK. Significance of galectin-3 expression in pulmonary non-small cell carcinoma. Korean J Pathol. 2006 10;40(5):326-332.
8. Kim HJ, Kim KH, Jang YD, Cho SH, Koh YW, Kim DW, et al. The expression of vascular endothelial growth factor (VEGF) and survivin in squamous cell carcinoma of larynx. Korean J Otolaryngol-Head Neck Surg. 2006 12;49(12):1188-1193.
9. Jung KY, Choi JO. Clinical significance of the expression of oncosuppressor gene protein and epidermal growth factor receptor in squamous cell carcinoma of the larynx. Korean J Otolaryngol-Head Neck Surg. 1993;36(5):990-1004.
10. Lee DY, Im DM, Do NY, Na HJ, Choi JS, Kim SH, et al. Study on immunohistochemical expression of p53, epidermal growth factor and c-erbB-2 in squamous cell carcinoma of the head and neck. Korean J Otolaryngol-Head Neck Surg. 2001 4;44(4):412-447.
11. Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994 2 25; 76(4):597-598. PMID: 8124704.
12. Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002 9;1572(2-3):232-254. PMID: 12223272.
14. Hirabayashi J, Kasai K. The family of metazoan metal-independent ╬▓-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993 8;3(4):297-304. PMID: 8400545.
15. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002 9 19; 1572(2-3):263-273. PMID: 12223274.
16. Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998 5;76(6):402-412. PMID: 9625297.
17. Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002 9 19; 1572(2-3):285-293. PMID: 12223276.
18. Haugen BR, Woodmansee WW, McDermott MT. Towards improving the utility of fine-needle aspiration biopsy for the diagnosis of thyroid tumours. Clin Endocrinol (Oxf). 2002 3;56(3):281-290. PMID: 11940037.
19. Bidon-Wagner N, Le Pennec JP. Human galectin-8 isoforms and cancer. Glycoconj J. 2004;19(7-9):557-563. PMID: 14758080.
20. Rechreche H, Mallo GV, Montalto G, Dagorn JC, Iovanna JL. Cloning and expression of the mRNA of human galectin-4, an S-type lectin down-regulated in colorectal cancer. Eur J Biochem. 1997 8 15; 248(1):225-230. PMID: 9310382.
21. Nagy N, Legendre H, Engels O, Andre S, Kaltner H, Wasano K, et al. Refined prognostic evaluation in colon carcinoma using immunohistochemical galectin fingerprinting. Cancer. 2003 4 15; 97(8):1849-1858. PMID: 12673710.
22. Levy Y, Arbel-Goren R, Hadari YR, Eshhar S, Ronen D, Elhanany E, et al. Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem. 2001 8 17; 276(33):31285-31295. PMID: 11371555.
23. Bidon N, Brichory F, Bourguet P, Le Pennec JP, Dazord L. Galectin-8: a complex sub-family of galectins (Review). Int J Mol Med. 2001 9;8(3):245-250. PMID: 11494049.
24. Danguy A, Rorive S, Decaestecker C, Bronckart Y, Kaltner H, Hadari YR, et al. Immunohistochemical profile of galectin-8 expression in benign and malignant tumors of epithelial, mesenchymatous and adipous origins, and of the nervous system. Histol Histopathol. 2001 7;16(3):861-868. PMID: 11510978.
25. Nagy N, Bronckart Y, Camby I, Legendre H, Lahm H, Kaltner H, et al. Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor. Gut. 2002 3;50(3):392-401. PMID: 11839721.
26. Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H, et al. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001 1;11(1):12-26. PMID: 11145198.
27. Bidon N, Brichory F, Hanash S, Bourguet P, Dazord L, Le Pennec JP. Two messenger RNAs and five isoforms for Po66-CBP, a galectin-8 homolog in a human lung carcinoma cell line. Gene. 2001 8 22; 274(1-2):253-262. PMID: 11675018.
28. Bassen R, Brichory F, Caulet-Maugendre S, Bidon N, Delaval P, Desrues B, et al. Expression of Po66-CBP, a type-8 galectin, in different healthy, tumoral and peritumoral tissues. Anticancer Res. 1999;NovŌĆōDec;19(6B):5429-5433. PMID: 10697573.
29. Henno S, Brichory F, Langanay T, Desrues B, Bidon N, Delaval P, et al. Expression of Po66-CBP, a galectin-8, in different types of primary and secondary broncho-pulmonary tumors. Oncol Rep. 2002;JanŌĆōFeb;9(1):177-180. PMID: 11748478.
30. Caulet-Maugendre S, Birolleau S, Corbineau H, Bassen R, Desrues B, Bidon N, et al. Immunohistochemical expression of the intracellular component of galectin-8 in squamous cell metaplasia of the bronchial epithelium in neoplastic and benign processes. Pathol Res Pract. 2001;197(12):797-801. PMID: 11795826.
31. Su ZZ, Lin J, Shen R, Fisher PE, Goldstein NI, Fisher PB. Surface-epitope masking and expression cloning identifies the human prostate carcinoma tumor antigen gene PCTA-1 a member of the galectin gene family. Proc Natl Acad Sci U S A. 1996 7 09; 93(14):7252-7257. PMID: 8692978.
32. Wollina U, Graefe T, Feldrappe S, Andre S, Wasano K, Kaltner H, et al. Galectin fingerprinting by immuno- and lectin histochemistry in cutaneous lymphoma. J Cancer Res Clin Oncol. 2002 2;128(2):103-110. PMID: 11862481.
33. Simon P, Decaestecker C, Choufani G, Delbrouck C, Danguy A, Salmon I, et al. The levels of retinoid RARbeta receptors correlate with galectin-1, -3 and -8 expression in human cholesteatomas. Hear Res. 2001 6;156(1-2):1-9. PMID: 11377877.
Fig.┬Ā1Immunohistochemical staining of galectin-8 in laryngeal squamous cell carcinoma shows intense immunoreactivity (arrows), and particularly in the stromal invasive region (LSAB method, counterstained by Mayer's hematoxylin, ├Ś200). 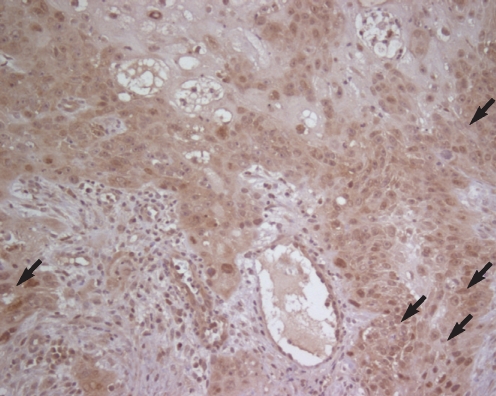
Fig.┬Ā2Immunohistochemical staining of galectin-8 in laryngeal squamous cell carcinoma. A strong galectin-8 expression (arrows) is identified in the squamous cell carcinoma (lower portion) as compared with the normal and dysplastic squamous epithelium (upper portion) (LSAB method, counterstained by Mayer's hematoxylin, ├Ś200). 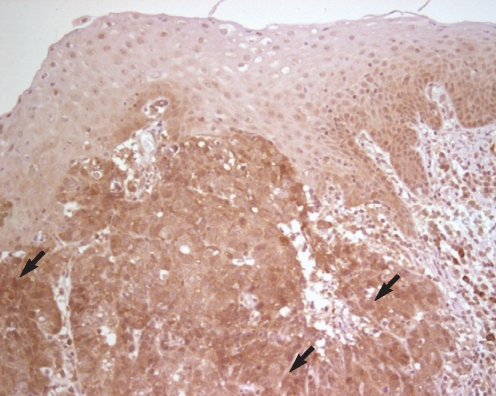
Fig.┬Ā3Galectin-8 encodes for a galectin with two homologous carbohydrate-binding regions. A schematic structure of galectin-8 is presented. Each box represents a putative carbohydrate-binding domain, linked by a 32 amino acid-long peptide. Also shown are selected invariant amino acids that are preserved in most of the galectins that have currently been analyzed. 
Table┬Ā1The clinical, epidemiologic and histologic characteristics of 77 patients with laryngeal squamous cell carcinoma (%) 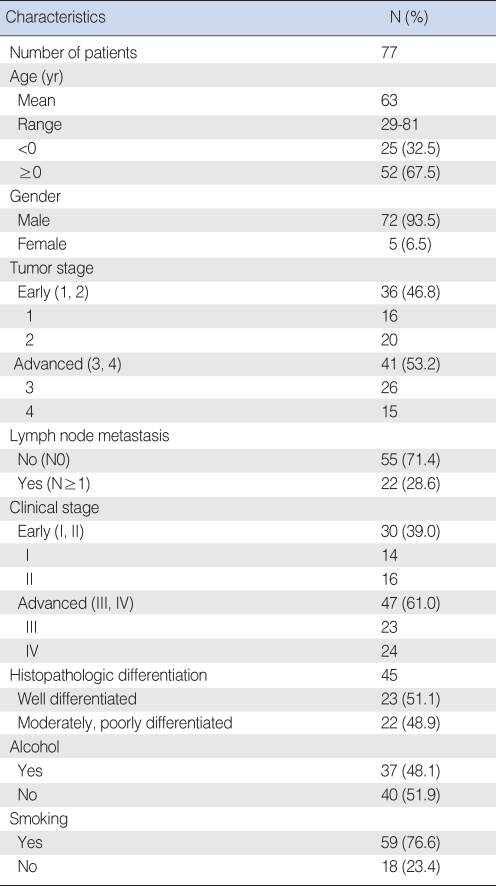
Table┬Ā2The immunohistochemical staining results for galectin-8 in laryngeal squamous cell carcinoma (%) 
Table┬Ā3The statistical analysis of the immunohistochemical galectin-8 expression according to the T-stage (%) 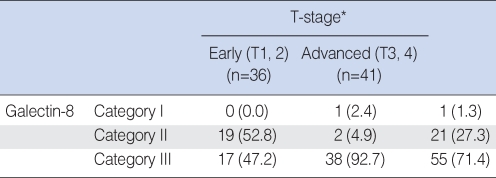
Table┬Ā4The statistical analysis of the immunohistochemical galectin-8 expression according to the nodal stage (%) 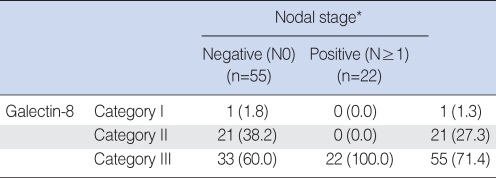
|
|
|||||||||||||||||||||||||||||||||||||||||