Parameters of Stromal Activation and Epithelial to Mesenchymal Transition as Predictive Biomarkers for Induction Chemotherapy in Patients With Locally Advanced Oral Cavity and Oropharyngeal Squamous Cell Cancer
Article information
Abstract
Objectives
Induction chemotherapy (IC) is likely to be effective for biologically distinct subgroups of oral cancer and biomarker development may lead to identification of those patients.
Methods
We evaluated immune cell infiltration, stroma formation and structure of the invasive front as well as the immunohistochemical expression of alpha smooth muscle actin (ASMA), CD163, E-cadherin, N-cadherin, and the laminin gamma 2 chain in pretreatment biopsy specimens and surgical resections after IC in 20 patients with locally advanced oral cancer who were treated in a prospective, ongoing, phase II trial on IC using docetaxel, cisplatin, and 5-fluorouracil (TPF).
Results
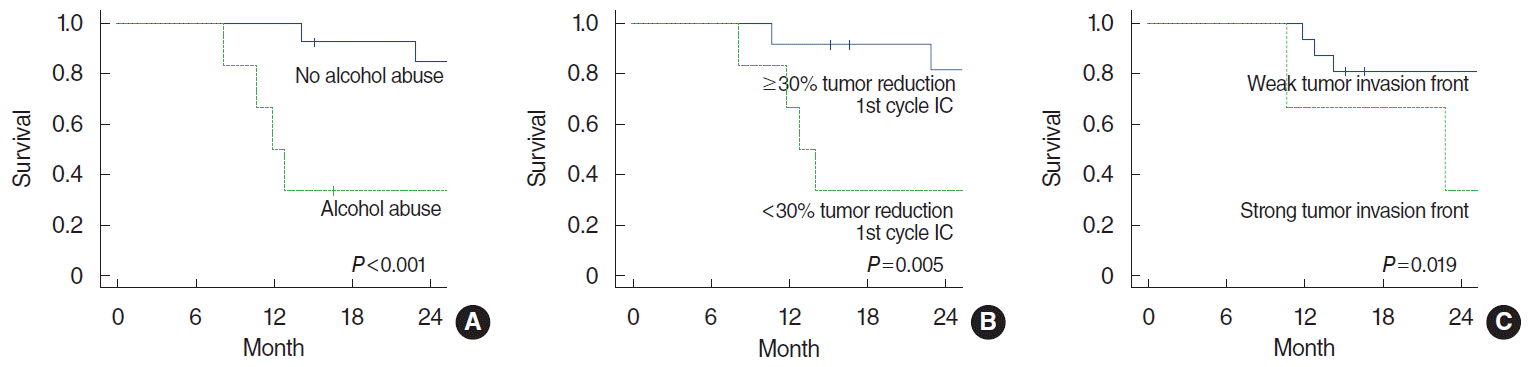
Significant negative prognostic factors for incomplete pathological tumor response to IC were alcohol abuse (P=0.032), cN+ (P=0.042), and <30% tumor reduction after first cycle of IC (P=0.034). Of the investigated histological parameters and biomarkers only a low membrane-bound expression of E-cadherin showed a trend to be associated with incomplete response to IC (P=0.061). Low expression of ASMA in stromal vessels and a strong tumor invasion front were significantly associated to tumor recurrence (P=0.024 and P=0.004, respectively). The median follow-up of all patients was 35 months. Alcohol abuse (P<0.001), <30% tumor reduction after first cycle of IC (P=0.005), and a strong tumor invasion front (P=0.019) were negative prognostic factors for overall survival.
Conclusion
A strong predictive biomarker among the investigated parameters for benefitting from TPF IC could not be found. The extent of the tumor invasion front was a negative prognostic marker for recurrence and survival in oral cancer treated by TPF IC followed by surgery and postoperative radiochemotherapy.
INTRODUCTION
As a component of multimodal therapy in locally advanced head and neck cancer, induction chemotherapy (IC) represents a strategy to reduce tumor burden and target distant metastases prior to definitive treatment [1]. The addition of taxanes to the cisplatin and 5-fluorouracil induction regimen (TPF) has significantly improved outcomes in comparison with cisplatin and 5-fluorouracil (PF) alone [2,3]. However, as a recent phase II trial (DeCIDE) and a phase III trial (PARADIGM) may have been underpowered to show a survival advantage for TPF induction followed by chemoradiotherapy versus chemoradiotherapy alone [4,5], there is an ongoing debate which subset of patients may benefit from TPF [6,7]. Recently, it has been shown that growth differentiation factor 15 (GDF15) expression can be used as a prognostic biomarker for oral squamous cell carcinoma, and as a predictive biomarker for benefitting from TPF IC [8]. Furthermore, patients with low annexin A1 expression or low p53 expression significantly profited more from TPF IC than patients with high expression of the these oncogenic factors [9,10].
However, a lower rate of distant metastatic disease was noted in the above mentioned DeCIDE study, suggesting that patients who are at high risk for metastatic disease may benefit from IC [7]. For epithelial malignancies, the epithelial-mesenchymal transition (EMT) is considered to be the crucial event in the metastatic process [11]. EMT is defined as loss of epithelial morphology and acquisition of migratory mesenchymal features, which allows the tumor cells to pass through the basement membrane and to travel to the site of metastasis formation. EMT is achieved by down-regulation of epithelial cell junction proteins like E-cadherin and for instance by de novo expression of mesenchymal proteins such as vimentin [11,12]. Furthermore, EMT is not only crucial for tumor biological behavior and progression of oral cancer. It seems to be also a cellular strategy for the development of drug resistance [13].
Stromal components, i.e., the microenvironment of the tumor, play a key role in the process of EMT [14]. Tumor-stroma cross talk is evidenced as a precondition for the development of the invasive tumor cell phenotype also in oral squamous cell carcinoma. This is accompanied by a fibroblast to myofibroblast transition also suggested as the main source of the so called carcinoma associated fibroblasts (CAFs). These alpha smooth muscle actin (ASMA) positive CAFs are able to modulate phenotype and signaling pathways of the carcinoma cells towards a more motile/aggressive stage. Indeed it could be shown that the expression level of ASMA is a marker for prognosis in oral squamous cell carcinoma (OSCC) [15,16]. Again, with respect to the crucial impact of CAFs on oral carcinoma cell phenotype, they also may play a critical role in modulating therapy sensitivity in head and neck cancer [17].
Up to now, the predictive value of the expression of stromal activation and EMT markers for IC efficacy is not investigated in detail. Hence, the present translational clinical study used a subset of patients of an ongoing prospective clinical phase II trial TISOC-1 (ClinicalTrials.gov; NCT01108042) to evaluate tumor stroma and EMT predictive biomarkers for better selection of patients for treatment regimens using TPF IC. Standard histology was used (1) to analyze the tumor stroma formation, inflammatory reaction, and mode of invasion. Immunohistochemistry was applied to (2) characterize the stromal activation using the stromal markers ASMA and CD163, and (3) to explore the extend of EMT using the EMT markers E-cadherin, N-cadherin and cytoplasmic laminin gamma 2 prior to IC and after IC.
MATERIALS AND METHODS
Patients
This translational study was part of an ongoing prospective multicenter phase I/II clinical trial (TISOC-1; ClinicalTrials.gov; NCT01108042; 72 patients included; last patient in, August 2013; last patient out, December 2015). The first 20 patients treated at the study center at the Jena University Hospital, Jena, took place in this translational study. The per-protocol follow-up of the 50th patient ended in January 2014. Therefore, it was possible to report the outcome of this translational study before the phase II clinical trial is ended. The protocol was approved by the ethics committee for human research at the Medical Faculty, Friedrich-Schiller-University Jena. All patients provided written informed consent before registration. Demographic and medical variables were measured by chart review. TNM staging was performed according to the American Joint Committee on Cancer (AJCC) cancer staging classification (2010). The chart review provided the basis to assess the tumor and treatment characteristics. Alcohol consumption was categorized into <25 g/day and >25 g/day. Patients were classified as smokers if they smoked cigarettes or quit smoking ≤3 months ago. All other patients were classified as nonsmokers.
Therapy
TISOC-1 studies the outcome of docetaxel, cisplatin und 5-fluorouracil (TPF) as IC prior to surgery and postoperative radiotherapy or radiochemotherapy in cavity of the mouth and oropharyngeal cancer [18]. Patients received a split-dose regime of TPF (30 mg/m2 docetaxel, 40 mg/m2 cisplatin, and 2,000 mg/m2 5-fluorouracil) on two day 1 and 8 per cycle for one or three 3-week cycles prior to surgery and postoperative radiotherapy or radiochemotherapy. Chemotherapy was applied with usual premedications, appropriate antiemetics and intravenous hydration. Tumor response was evaluated on day 21 using a clinical examination with endoscopy of the primary tumor. Tumor response to chemotherapy was defined by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and as a reduction of the tumor volume ≥30%. All responders received two more cycles of split-TPF, i.e., responders underwent surgery after three cycles and nonresponders after one cycle of CT whereas nonresponders underwent surgery after one cycle of surgery. Surgery was performed within 3–5 weeks after chemotherapy. Surgery was performed within the original tumor margins prior to TPF IC. Ipsilateral neck dissection was obligatory. For tumors crossing the midline a contralateral neck dissection was recommended. Start of postoperative radiotherapy was recommended not later than 8 weeks after last surgery. Criteria for high risk patients were: R1 resection, resection margins <5 mm, extracapsular spreading, perineural tumor growth, vascular tumor embolus, lymph node metastasis in level IV or V, >2 positive lymph nodes. All other patients were classified as low risk patients. Low risk patients received postoperative radiotherapy (3-D conformal or with intensity modulated radiotherapy). High risk patients received radiochemotherapy with cisplatin (20 mg/m2) five times in week 1 and week 5 of radiotherapy.
Histology and immunohistochemistry
Pretreatment biopsies and resected surgical samples of the primary tumor were collected, formalin-fixed, and paraffin-embedded. For histology and immunohistochemistry 4 μm paraffin sections were used. Standard histopathology was performed with hematoxylin and eosin staining. For immunohistochemistry sections were incubated with the monoclonal antibodies against ASMA (1:600; clone 1A4; DakoCytomation, Hamburg, Germany), E-cadherin (1:500; clone NCH-38; DakoCytomation), N-cadherin (1:100; clone 6G11; DakoCytomation), laminin gamma 2 (1:3,000; clone D4B5; Chemicon/Merck Chemicals GmbH, Schwalbach, Germany), or CD163 (1:500; clone 10D6; Novocastra/Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany) for 1 hour at room temperature, respectively. Negative control was performed by using tris-buffered saline (TBS) instead of primary antibodies. For detection of the primary antibodies by means of bright field microscopy, the Dako REAL Detection System and, in case of CD163, the Envision Flex Detection System (both from DakoCytomation) were used as recommended by the manufacturer. All samples were evaluated in the light microscope (Axioscop-2; Zeiss; Jena, Germany) independently by two investigators (JG and AB), and then jointly for consensus. The investigators were blinded to the clinicopathologic data at the time of the evaluation. Evaluation of the tumor stroma, inflammatory reaction, and mode of invasion was performed using the hematoxylin and eosin stained slides according to [19,20]. Both, histological parameters as well as immunohistochemical detection levels of the positivity of the different antibodies were semiquantitatively assessed using a 0–3 scoring system (Supplementary Table 1). For the statistical evaluation, the scores 0 and 1 were summarized as “no/low” and the scores 2 and 3 as “high.”
Statistical analysis
Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Co., Armonk, NY, USA). To compare patient and tumor characteristics to the rate of pathological complete response to IC, recurrence, and death, Pearson chi-square test was used. Therefore, if necessary, all data of these parameters were dichotomized to categorical data using median values as separators. Overall survival was calculated by the Kaplan-Meier method and differences of survival were compared by the log-rank test. Due to the small sample size, a multivariate analysis was not appropriate. For all statistical tests, significance was two-sided and set to P<0.05.
RESULTS
Subjects
Details on the patients’ characteristics are presented in Table 1. Two thirds of the patients were male and smokers. Median age was 56 years. One third was alcohol dependent and most patients had stage IV tumors. IC was stopped after the first cycle because of insufficient response <30% clinical tumor reduction in six patients (30%) and because of toxicity on two patients (10%). All other 12 patients (60%) received three cycles of IC.
Tumor stroma and EMT before and after induction chemotherapy
An overview of the histological and immunohistological results on the tumor and tumor stroma characteristics prior to treatment is shown in Supplementary Table 2. Histological and immunohistochemical examples of the tumor stroma and EMT activation are given in Fig. 1. The majority of the tumors showed a high laminin gamma 2 expression, no/low ASMA expression in the stroma but high AMSA expression in the stromal vessels, no/low CD163 expression, and no/low membrane-bound and cytoplasmatic E-cadherin expression. N-cadherin expression was absent or low in all patients. Stroma formation was strong in one thirds of patients. Stromal inflammation was higher in about half of patients, whereas the invasion front structure was “low” (pushing borders) in nearly all patients.

Histological and immunohistological examples of tumor stroma and epithelial-mesenchymal transition activation in oral cancer prior to induction chemotherapy. (A) H&E, case 11; mode of invasion, score 3 (detached island/disseminated); Inflammation, score 2 (moderate). (B) H&E, case 3; mode of invasion, score 1 (pushing borders); inflammation, score 2–3 (moderate – distinct inflammatory reaction). (C) E-cadherin immunohistochemistry (IH), case 21; membranous positivity, score 3 (positive >30% tumor area)/red staining. (D) Laminin gamma 2 chain IH, case 20; cytoplasmic positivity, score 3 (tumor cells more or less all positive)/red staining. (E) Alpha smooth muscle actin IH, case 3; stromal positivity, score 2–3 (distinct stroma positivity, note the accentuation of the tumor borders)/red staining. (F) CD163 IH, case 3; CD163 positivity, score 2 (many positive cells detectable)/brown staining.
The results of the examination of the surgical specimen after IC and curative surgery are summarized in Supplementary Table 3. IC led to downstaging of the primary tumor in most cases but not of the lymph node metastases. A complete pathological response was seen in seven patients (35%). Laminin gamma 2 expression decreased in half of the patients, whereas E-Cadherin expression was unchanged in more than half of the patients. N-cadherin expression was predominantly unchanged after IC. Stroma formation, inflammation and mode of invasion were unchanged in most of the patients with residual tumor after IC.
Tumor stroma and EMT as prognostic biomarkers
The negative prognostic role of all relevant parameters concerning no pathological complete response, tumor recurrence and death of the patients is presented in Table 2. Significant negative prognostic factors for incomplete tumor response to IC were alcohol abuse (P=0.032), cN+ (P=0.042), and no significant (<30%) tumor reduction after first cycle of IC (P=0.034). Of the investigated biomarkers only a low membrane-bound expression of E-cadherin showed a trend to be associated with incomplete response to IC (P=0.061). Low expression of ASMA in the stromal vessels and a “high” tumor invasion front (disseminated invasion pattern) were significantly associated to tumor recurrence (P=0.024 and P=0.004, respectively). Alcohol abuse (P=0.010), no relevant tumor reduction after first cycle of IC (P=0.019), and again a strong tumor invasion front (P= 0.021) were correlated to higher probability of patient’s death after cancer treatment. A subanalysis of only the patients receiving 3 cycles of IC confirmed that a strong tumor invasion front was correlated to higher risk of recurrence and death (P=0.005 and P=0.001, respectively) (Supplementary Table 4). Furthermore, a low expression of membrane-bound E-cadherin was related in this subset of patients to higher risk of incomplete response to IC (P=0.046).
The median follow-up of all patients and patients alive was 35 months and 49 months, respectively. The overall survival analyses are shown in Supplementary Table 5. In correspondence to the results presented above, alcohol abuse (P<0.001), no relevant tumor reduction after first cycle of IC (P=0.005), and a “high” tumor invasion front (P=0.019) were negative prognostic factors for overall survival (Fig. 2).
DISCUSSION
To our knowledge, the presented analysis is a small but first and prospective evaluation of the effect of the tumor stroma and EMT on IC in patients with cancer of the oral cavity and the oropharynx. In most therapy strategies and studies using IC, IC with TPF is normally followed by radiotherapy or radiochemotherapy, i.e., surgical specimens after IC are not available [2]. That means that in such studies prognostic biomarkers, for instance, for overall survival in a therapy setting using TPF IC can be studied but not the predictive value of IC on the pathological tumor response. For instance, it was shown that the expression of the biomarkers beta tubulin II, glutathione S-transferase, p53, or B-cell lymphoma 2 in the head and neck tumor treated with TPF IC is associated with worse outcome [21,22]. High annexin A1 expression is linked to worse survival after TPF IC in oral cancer [9]. This may be due to its role in plasma membrane repair after membrane injury which is caused by mechanical stress during cell invasion and metastasis [23]. However, all these markers are not directly linked to the tumor stroma or EMT.
Beside the present study, there is only one other study published which had access to a prospective clinical trial on TPF IC following by surgery, i.e., access to specimens after IC [8]. This Chinese study showed that GDF15 overexpression in the tumors was negative predictive marker for benefitting from TPF IC. The tumor stroma was not analyzed [8]. With respect to tumor stroma and EMT, we found that the majority of the patients showed a high cytoplasmic laminin gamma 2 chain expression in tumor cells prior to treatment. In line with that, it is well known that laminin gamma 2 as an EMT marker is correlated with the invasiveness of the tumor. The laminin gamma 2 expression is also correlated to the ASMA expression speaking well for the important role of stromal myofibroblast transdifferentiation for cancer cell phenotype transition and vice versa [24,25]. In the present study only a quarter of the patients showed a high ASMA expression in the stroma but the majority showed a high ASMA expression in the stromal vessels. Anyhow, in accordance to the literature the degree of stromal ASMA was significantly correlated to the laminin gamma 2 expression (data not shown) [25]. Stromal ASMA expression as a sign of CAF development is an indirect marker of the EMT activity of the tumor and therefore a known risk factor for metastasis formation [14,17]. In the present study, stromal expression of CD163 was low in most cases. CD163 is a marker of tumor stroma-associated M2 macrophages. Increased expression of CD163 was significantly associated with a poor overall survival in several cancers but only one study has confirmed that for oral cancer [26]. We could not confirm this role for CD163 in the present study although at least about half of the patients in the present study showed tumor stroma with strong inflammatory reaction. E-cadherin and N-cadherin, opposite acting EMT biomarkers, predominately showed low expression profiles in untreated tumors. Loss of E-cadherin, i.e., low E-cadherin expression and less control over local cell adhesion in the tumor stroma is correlated to worse outcome [11,27]. Inversely high N-cadherin expression is correlated to worse outcome, because it increases extracellular matrix-associated proteolytic activity to facilitate invasiveness in oral tumor development [28]. Overall, most of the observed EMT and stromal marker profiles correlated to data from the literature. Also vascular ASMA showed a significant negative correlation to recurrence in the present investigation. Low vessel associated ASMA positivity may reflect a higher percentage of immature vessels and therefore a higher angiogenetic potential of the tumor. Although critically discussed, high microvessel density was shown to be a risk factor for recurrence in OSCC [29,30]. Nevertheless, classical histological evaluation of the tumor invasion front into the tumor stroma was highly predictive for worse survival as it has also been shown in other recent studies [31,32]. We therefore hypothesize that the influence of IC on the tumor stroma might be a reason why TPF IC seems to reduce the cumulative incidence of distant metastasis [5].
Concerning the prediction of complete pathological response to TPF IC, no significant stromal marker could be detected in the present study. When considering all patients, only low E-cadherin expression showed a statistical trend to be correlated to incomplete pathological response. In the subset of patients receiving all three cycles of IC, low E-cadherin expression was significantly associated to higher probability of incomplete pathological response. Low E-cadherin may represent an ongoing mesenchymal phenotype transition toward a more therapy resistant phenotype. In the only other study with surgical resection specimens available after TPF IC (there is even no other study published analyzing surgical specimens after any kind of IC in oral cancer), the authors did not performed any analysis related to the pathological tumor response [8]. Interestingly, the individual stromal biomarker expression profiles did not change in the majority of the patients. This might explain why alteration of the stroma after TPF IC did not have any predictive value on pathological tumor response, recurrence or death of the patients.
It is important to address the limitations of the present study. Although it is the largest study published so far reporting on tumor stroma reaction prior to IC compared to after IC, the sample was small with 20 patients. This might explain why some well-known negative prognostic factors for overall survival like T and N classification did not reach statistical significance although the related Kaplan-Meier curves take a completely different and separated course. Against this background the fact that a disseminated mode of invasion was shown to be a significant negative risk factor for overall survival is all the more important. Nevertheless, the sample size was too small to allow a meaningful multivariate analysis. The pretreatment histological and immunohistological assessment of the tumors was based on several representative biopsies from different subsites of the tumor but not based on serial sections from the complete specimen as it was possible for the postoperative assessment. Therefore, we finally cannot rule out that some variability of the pretreatment results is related to a biopsy selection bias.
It seems to be worthwhile to extent the research on the relation between IC and tumor stroma and EMT in head and neck cancer patients on larger study samples. Furthermore, as TPF IC seems to be most advantage in nonoropharyngeal sites, we would like to explore the role of stromal biomarkers in nonoropharyngeal tumors, for instance in laryngeal or hypopharyngeal cancer [4]. Finally, as IC with TPF incorporating cetuximab appears to yield a greater response than TPF, it would be interest to investigate stromal biomarkers after IC strategies including cetuximab [33].
HIGHLIGHTS
▪ The effect of induction chemotherapy on the tumor stroma of oral cancer was analyzed.
▪ A strong predictive biomarker for benefitting from induction chemotherapy was not found.
▪ A strong tumor invasion front was a negative prognosticator for recurrence and survival.
Notes
No potential conflict of interest relevant to this article was reported.
Supplementary Material
Scoring system for semiquantitative assessment of the investigated parameters
Pre-treatment histopathology and immunohistochemistry results (n=20)
Histopathology and immunohistochemistry after induction chemotherapy and surgery (n=20)
Subanalysis of patients with three cycles of induction chemotherapy: prognostic factor for no pathological complete remission, tumor recurrence, death, and overall survival (n=12)
Influence of baseline tumor parameters on overall survival (n=20)