INTRODUCTION
Medial olivocochlear (MOC) efferent system has two presumed roles related with cochlear mechanics in noise. The most commonly accepted is that it facilitates the detection of signals in background noise by attenuating the gain of the cochlear amplifier to physiological stimuli [1-3]. Efferent fibers have also been documented to protect the ear against acoustic injury. Experimental studies proposed that the strength of the MOC reflex (MOCR) may be the indicator of noise susceptibility [4]. MOC neurons that originate in the medial portion of the superior olive and project to the outer hair cells (OHCs) constitute the efferent arm of the MOCR pathway [5,6]. MOC effects are reflected in changes of otoacoustic emissions (OAEs) in the presence of ipsilateral and/or contralateral acoustic stimulation (CAS). Distortion-product OAE (DPOAE)-based contralateral MOCR provides the test of choice for the integrity and strength of the MOC system [2,6].
Baso-apical gradients exist in various cochlear structures along the length of the cochlea [7,8]. It is also well known that the cochlea has a spatial gradient in the susceptibility to noise with the most vulnerable OHCs at the basal end [9]. In addition, MOC neurons demonstrate sharp frequency tuning curves with tonotopicity that is found consistently within the auditory pathways [5,10]. Maison et al. [11,12] have reported longitudinal gradients of MOC innervation density and the strength of efferent effects along the cochlear spiral in the mouse. This study aimed to confirm the cochlear regional differentials in the function and morphology of the MOC system and to address the functional implications of the regional MOC efferent terminals (ETs) in the mouse cochlea.
MATERIALS AND METHODS
Four-week-old male CBA/J mice were purchased from Orient Bio (Sungnam, Korea) and kept in our animal facility. Experiments were performed on both ears in 8 mice. All animal procedures followed the national ethical guidelines and relevant laws. The experimental protocol was approved by the Animal Care and Use Committee of the Catholic University of Korea College of Medicine.
Auditory tests
Mice were anesthetized with intraperitoneal injection of a mixture of zolazepam-tiletamine (5 mg/kg) and xylazine (5 mg/kg). All tests were conducted in a custom-made sound-attenuated chamber for mice. For hearing screening, auditory brainstem responses (ABRs) to click (0.1 msec, 19.3/sec) and tone bursts (8/16/32 kHz, 1.5 msec, 21.1/sec) were recorded using SmartEP fitted with high-frequency transducers and high-frequency software ver. 2.33 (Intelligent Hearing Systems, Miami, FL, USA). Test stimuli were delivered to the ear canal through a miniature insert earphone from 90 dB to 10 dB sound pressure level (SPL) in 5 dB steps. Electrical potentials in the first 12 msec after the stimuli were sampled via subdermal stainless steel needle electrodes at the vertex (active) and pinna (reference) with 0.1ŌĆō3 kHz bandpass filtering, and averaged for 256 sweeps. Thresholds were defined as the lowest stimulus intensity where the typical wave pattern was still identified. SmartOAE ver. 4.26 (Intelligent Hearing Systems) was used to measure the 2f1-f2 DPOAE in the ear canal and to obtain DP-gram that displays DPOAE amplitude plotted as a function of f2 frequency. DPOAE recordings were made for f2 frequencies from 6.5 to 35 kHz at 8 points per octave using primary tone paradigm set as follows: L1=65 dB, L2=55 dB SPL, and f1/f2=1.22. Stimulus signals were passed through ER-10B+ probe microphone inserted into the ear canal in conjunction with two types of transducers: ER-2 transducer (Etymotic Research, Elk Grove Village, IL, USA) for frequencies from 6 to 16 kHz and high-frequency transducer (Intelligent Hearing Systems) for frequencies from 16 to 32 kHz. The output was sampled at 128 kHz using a 16-bit A/D converter. Four blocks were acquired in total and each block consisted of 32 sweeps. Tests were performed under two stimulus conditions of the opposite ear with and without CAS. Broadband noise MOC elicitor was presented to the contralateral ear at 55 dB SPL that does not evoke stapedial reflex in mammals [13].
Immunostaining and quantification of MOC terminals
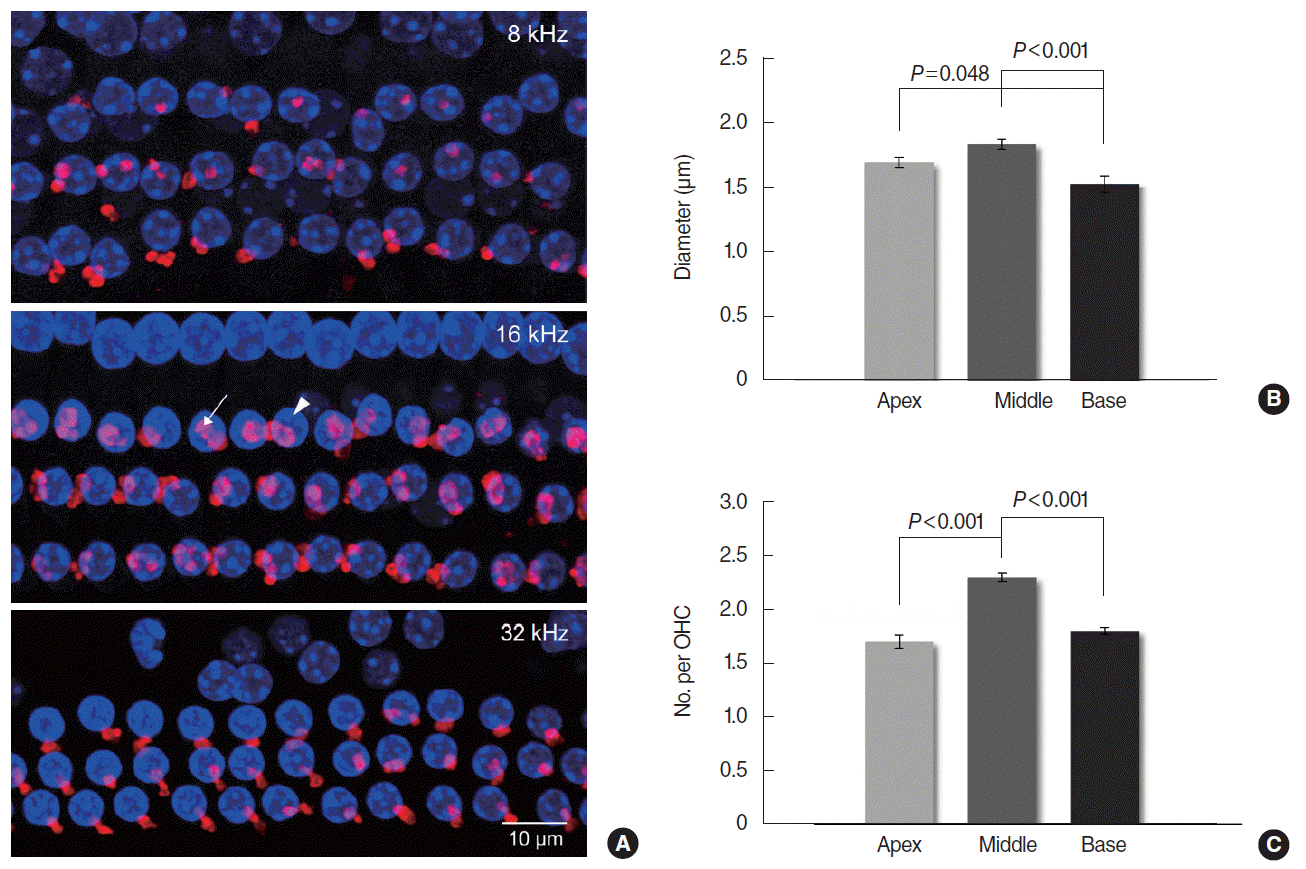
Mice were anesthetized and killed to harvest the cochleas, which were processed for whole mount immunofluorescent staining and confocal microscopy. The cochlea was perfused and fixed with 2% paraformaldehyde, and then decalcified in ethylenediaminetetraacetic acid (EDTA) for 2ŌĆō4 days. The lateral wall, ReissnerŌĆÖs membrane, and tectorial membrane were removed in order after the otic capsule was opened. In whole mount cochleas, the ETs were made visible by immunofluorescent staining for ╬▒-synuclein, an efferent synaptic vesicular protein. Specimens were blocked in 5% normal goat serum and 0.3% Triton X-100 for 1 hour, and incubated with 1:100 anti-╬▒-synuclein antibody (purified mouse anti-╬▒-synuclein, BD Biosciences, San Jose, CA, USA) at 4┬░C overnight in a humid chamber. They were rinsed and incubated with 1:4,000 fluorescent secondary antibody (Alexa Fluor 555 goat anti-mouse IgG, Thermo Fisher Scientific, Waltham, MA, USA) for 2 hours. After the secondary antibodies had been removed, specimens were exposed to Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) which allows visualization of the OHC nuclei. The frequency regions of interest were localized along the cochlear spiral at a low magnification according to a place-frequency map of mouse cochlea published previously [14]. Eighteen percent distance from the apex designated 8 kHz region, 43% distance designated 16 kHz, and 68% distance designated 32 kHz. Stained whole mounts were examined by confocal laser scanning microscopy (LSM 510 Meta, Carl Zeiss, Jena, Germany). As the ET-OHC synapses are located at different heights, a confocal z-stack was obtained from each region of the cochlea. Approximately 30 series of images were acquired per a region by focusing from top to bottom with a 0.6 ╬╝m step in the z-axis through the whole mount specimen under a magnification of 1,000. The acquired z-plane images were reconstructed using IPLab software (BioVision Technologies Inc., Exton, PA, USA) to create a single z-stack image. The captured images were imported into Image J software (National Institutes of Health, Bethesda, MD, USA). For quantitative morphometric analysis, two indices were used: the ET diameter and the number of ETs per OHC. The diameters of all visible ETs in a single image were measured and averaged. The number of all ETs (range, 44 to 91) was counted and divided by the number of OHCs (range, 31 to 39) shown in that image.
Data processing and statistical analysis
DPOAE amplitude was measured from the noise floor at each test frequency. Data points of an amplitude less than 6 dB were rejected. A DPOAE change by the MOC effect was defined as CAS-on amplitude minus baseline amplitude. Negative changes indicated suppression and positive changes, enhancement. The DPOAE change was normalized to the baseline amplitude and termed suppression ratio as the measure of MOCR strength at a valid data point. F2 frequencies were grouped into three ranges based on the same mouse place-frequency map: the low frequencies (LF) included 6,573ŌĆō10,779 Hz, which corresponds approximately to a <30 percent distance region from the apex; the middle frequencies (MF), 11,904ŌĆō23,592 Hz, a 30ŌĆō60 percent distance region; the high frequencies (HF), 26,048ŌĆō35,056 kHz, a >60 percent distance region. Within each frequency range, the suppression ratios at all data points from 16 ears were averaged. Statistical analysis was done using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) at a two-tailed significance level of 0.05. Two way ANOVA was performed in advance of every statistical analysis to rule out any interaction between the group and the side of the tested ears. The suppression ratios were compared across the three frequency ranges. The morphometric indices of the ETs were compared across the three regions of the cochlea. One way ANOVA and post hoc TukeyŌĆÖs test were performed after Shapiro-Wilk test for normal distribution and LeveneŌĆÖs test for the equality of variances. Otherwise, Kruskal-Wallis test and post hoc Mann-Whitney U-test were applied with BonferroniŌĆÖs correction.
RESULTS
DPOAE-based MOCR activity
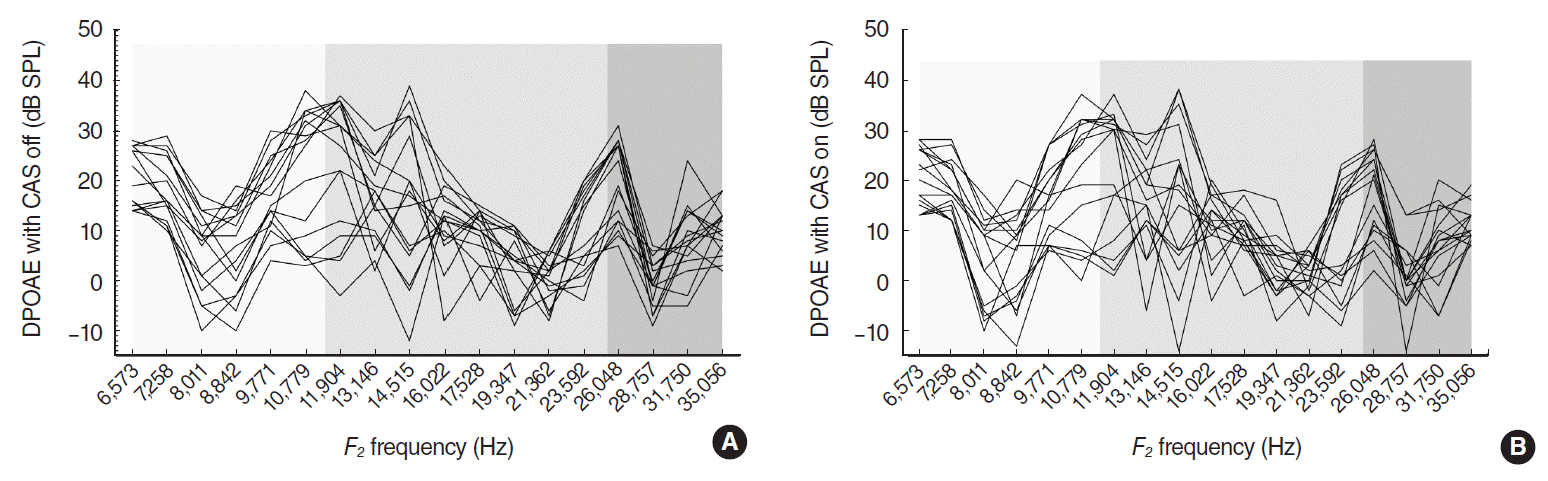
ABR thresholds at click, 8, 16, and 32 kHz tone bursts were 16.3┬▒0.6 (mean┬▒standard error of mean [SE]), 16.3┬▒0.6, 18.4┬▒0.9, and 19.4┬▒0.8 dB SPL respectively. All experimental mice had good hearing with ABR thresholds less than 20 dB SPL at the test stimuli on both ears. DP-grams obtained from 16 ears displayed a consistent pattern of amplitude variations with alternating peaks and dips in quiet, and in the presence of CAS (Fig. 1). There were no statistical differences in DPOAE levels between the right and left ears: 12.4┬▒7.7 dB SPL for the right and 11.8┬▒7.5 dB SPL for the left ears (grand mean of the mean DPOAEs at test frequencies┬▒standard deviation). DPOAE amplitudes were in the range of ŌĆō12 to 39 dB SPL without CAS, and ŌĆō14 to 38 dB SPL with CAS. Valid data points for CAS were 204 (71%) out of 288 (18 frequencies in 16 ears): 78, 84, and 42 from LF, MF, and HF respectively. CAS-evoked DPOAE changes exhibited both suppressions and enhancements ranged from ŌĆō16 to 11 dB SPL (Fig. 2A). Mean (SE) suppression ratio in LF/MF/HF was 0.19 (0.02)/0.39 (0.06)/0.42 (0.09) from 39/49/24 suppression points. Significant differences were shown between LF and MF (P=0.012), and between LF and HF (P=0.005) (Fig. 2B).
Morphometry of MOC efferent terminals
Whole mount organ of Corti labeled with anti-╬▒-synuclein showed clustered MOC ETs in the OHC area (Fig. 3A). Mean (SE) diameters of MOC terminals were 1.70 (0.04), 1.84 (0.04), and 1.53 (0.06) ╬╝m in the 8, 16, and 32 kHz regions respectively with significant differences between the 16 and 32 kHz (P<0.001), and between the 8 and 32 kHz regions (P=0.048) (Fig. 3B). Mean (SE) numbers of MOC terminals per OHC were 1.7 (0.06), 2.3 (0.04), and 1.8 (0.03) in the 8, 16, and 32 kHz regions respectively with significant differences between the 8 and 16 kHz (P<0.001), and between the 16 and 32 kHz regions (P<0.001) (Fig. 3C).
DISCUSSION
We performed DPOAE-based MOCR test and microscopic quantification of the MOC terminals in mice to investigate the regional differentials in MOC function and morphology along the cochlea and to identify the relationship between them. The DP-grams at 8 points/octave showed DPOAE fine structures. Contralateral MOCR resulted in both suppressions and enhancements of DPOAEs, but suppressions occurred more frequently and exhibited larger amplitudes, as reported in the previous literatures [15,16]. The DPOAE is a mixed emission generated by two different mechanisms in the cochlea: nonlinear distortion from f2 location and linear reflection from 2f1-f2 location. The vector combination of distortion and reflection components gives rise to DPOAE fine structure [17,18]. However, phase interference between two components can confound the interpretation of MOC effects on DPOAE: the out-of-phase cancellation at a fine-structure dip is abruptly released by contralateral noise through the phase shift in the reflection component, producing an increase in DPOAE level [19]. Therefore, we limited the index of MOCR activity to ŌĆśsuppressionŌĆÖ only in order to reject possible artifact ŌĆśenhancementŌĆÖ of DPOAE.
The size and number of the MOC terminals were the most remarkable in the 16 kHz region, which was similar with the previous studies. Maison et al. [11] have documented that longitudinal gradients of MOC terminals and the suppression of compound action potential peaked in the middle of the cochlear spiral near the 10 kHz region in the mouse, which indicates the correlation between the function and morphology of the MOC system. They have also reported that the average strength of shock-evoked DPOAE suppression was greatest in the middle (11ŌĆō22 kHz) of the test tones used (5.6ŌĆō45.2 kHz in half-octave steps) [12]. In our study, contralateral sound-evoked DPOAE suppression ratios measured at 8 frequencies/octave were significantly larger in MF (11.9ŌĆō23.6 kHz) and HF (26.0ŌĆō35.1 kHz) than in LF (6.6ŌĆō10.8 kHz) without difference between MF and HF.
Mice possess short cochleas and hear tones from 1 to near 100 kHz. The upper frequency limit for human hearing is approximately 20 kHz. The highest test frequency at 32 kHz region in mouse cochlea corresponds roughly to 8 kHz region in human cochlea [20]. Muller et al. [21] have documented that mice have a hearing differential across the audible range with the best ABR thresholds at a middle frequency band from 11 to 16 kHz. Also in humans, the middle frequencies from 0.5 to 2 kHz contribute mainly to speech perception. In our data, most of the DPOAE peaks were in the range of 10.8 to 14.5 kHz. It is notable that the best hearing frequencies in mice coincided approximately with the best frequencies for DPOAEs, MOCR, and the MOC ETs.
Overall, the middle cochlear region expressed large, clustered MOC ETs with strong MOCR, the base expressed small, less clustered MOC ETs with strong MOCR, and the apex expressed large, but less clustered MOC ETs with weak MOCR in mice. The base showed no correlation between the function and morphology of the MOC system. Weak MOCR with small number of MOC ETs in the apex may be related with the fact that mice perform poorly in the low frequencies [8]. However, strong MOCR in spite of inferior MOC morphology in the cochlear base may indicate the demand for ŌĆśprotection from noise traumaŌĆÖ in the high frequencies. The superior MOC activity and morphology of the middle region may indicate the weight of ŌĆśsignal detection in noiseŌĆÖ in the best hearing frequencies or another unknown efferent function beyond MOCR.
In conclusion, the mouse cochlea demonstrated regional differentials in the function and morphology of the MOC system. Strong MOCR along with superior MOC morphology in the middle frequency region may contribute to ŌĆśsignal detection in noise,ŌĆÖ the primary efferent function, in the best hearing frequencies. Strong MOCR in spite of inferior MOC morphology in the base may reflect the importance of ŌĆśefferent protection from noise traumaŌĆÖ in the high frequencies.
HIGHLIGHTS
Ō¢¬ The mouse cochlea demonstrated regional differentials in the function and morphology of the medial olivocochlear (MOC) system.
Ō¢¬ The middle cochlear region expressed large, clustered MOC terminals with strong MOC reflex (MOCR) in mice.
Ō¢¬ The base expressed small, less clustered MOC terminals with strong MOCR.
Ō¢¬ The apex expressed large, but less clustered MOC terminals with weak MOCR.