INTRODUCTION
The treatment for scar in a tubal structure, such as laryngeal, tracheal and nasal stenosis, remains one of the most difficult problems in the field of head and neck surgery. Scar formation and restenosis remain the main causes of failure in the surgical management of airway stenosis. Several topical or injected drugs (such as triamcinolone acetonide, 5-fluorouracil and mitomycin-C) have been widely used to overcome scarring and they achieve some symptomatic relief, but the final results for scar reduction or resolution are not always satisfactory (1-4). Mitomycin-C has both antineoplastic and antiproliferative properties. Its antineoplastic activity is similar to that of the alkylating agents, with their ability to cross-link DNA and inhibit both RNA synthesis and protein synthesis (5, 6). The antiproliferative action of mitomycin-C can inhibit the proliferative activity of fibroblasts (7). The canine vocal folds treated with topical mitomycin-C after microflap excision showed fewer fibroblasts and less collagen deposition in the lamina propria with reduced mucosal waves (8).
In the recent years, the use of topical and injected mitomycin-C has been proposed as a treatment option for laryngeal stenosis, but there are still insufficient experimental and clinical studies on its short and long-term effects and safety. Severe side effects have been reported, such as medullary aplasia and necrosis of the subcutaneous tissue at the injection site, following the administration of intravenous mitomycin-C at a total dose greater than 50 mg (9, 10). There have been reports of complications and a compromised airway (e.g., partial or total airway obstruction) related to the use of mitomycin-C for the treatment of glottis and subglottic stenosis (11).
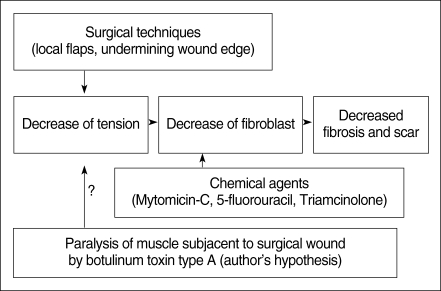
Tension is one of the chief factors that determine the degree of scar formation. Excessive tension on tissues has to be avoided in wound management because it compromises blood flow and increases the fibroplastic response. Various surgical techniques, such as placing scars in line with the relaxed skin tension lines, using local flaps or undermining the wound edges, have been applied to reduce excessive tension of an incision (12). A new method to minimize the underlying muscle contracture would be a desirable therapeutic modality for the healing of cutaneous wounds.
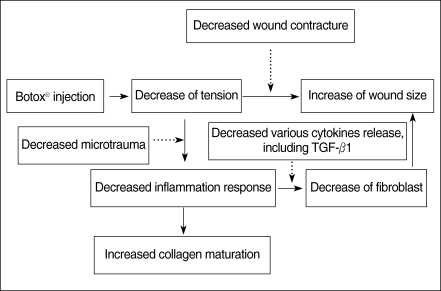
Botulinum toxin type A (Botox®, Allergan, Irvine, CA, USA) is known to be a potent neurotoxin that produces flaccid paralysis of the striated muscle for a period of 2 to 6 months; it has been safely used in humans in the clinical field for more than 20 yr. Its application to the ENT field provides a useful tool to treat spasmodic dysphonia, autonomic dysfunction, Frey syndrome, hemifacial spasm or synkinesis, and hyperfunctional lines (13, 14). Gassner and Sherris (15) reported that the chemoimmobilization of facial wounds with Botox improved the cosmetic appearance. So, we hypothesized that a Botox-induced paralysis of the musculature subjacent to the surgical wound with a skin defect would minimize the repetitive tensile forces on the wound edges, and this would result in a decreased fibroplastic response and fibrosis of the wound (Fig. 1). This study was designed to investigate the antifibroblastic effect of Botox on wound healing in a rat surgical wound model.
MATERIALS AND METHODS
Surgical wounds of the experimental animal
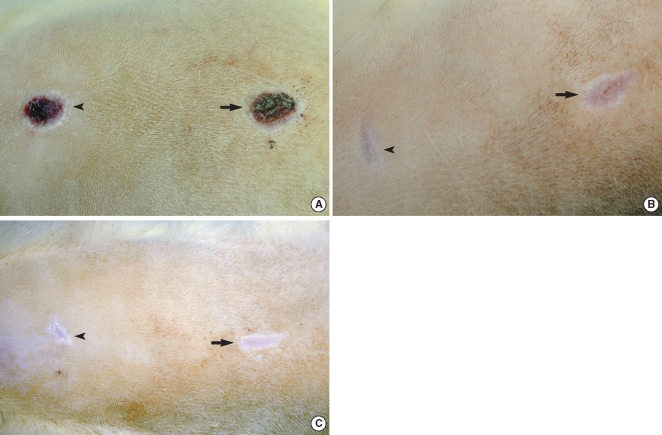
Fifteen male Wistar rats aged 120 to 150 days with weights that ranged from 350-410 g were used. They were anesthetized by consecutive intraperitoneal injections of Rumpune® (20 mg/kg, Bayer Korea, Seoul, Korea) and Ketamine® (100 mg/kg, Yuhan Company, Seoul, Korea). The animal care, housing and surgery followed the guidelines of the Animal Experiment Committee, Pusan National University Hospital. After each rat was shaved and two identical circular areas were prepared on the dorsum of each rat, and the distance between the areas was approximately 4 cm. The skin and subcutaneous tissue were then excised, but the deep muscle was preserved at the base of the defect. The diameter of the wound was 1.2 cm (Fig. 2). One of the wounds was selected at random and it was treated with Botox. Botox (10 U, 0.5 mL) was injected into the deep musculature subjacent to the wound immediately after the surgery. As a control, normal saline (0.5 mL) was injected into the other wound.
Assessment of wound size
Visual assessment of the state of the wound healing was done weekly by two otolaryngologists. They measured the lengths of the major axis and the minor axis of the wound every week with a ruler. The measurement of the area of the wound was calculated by the formula for an ellipse ([0.5 × the length of the major axis] [0.5 × the length of the minor axis] [π]) (16).
Histological assessment of wound healing
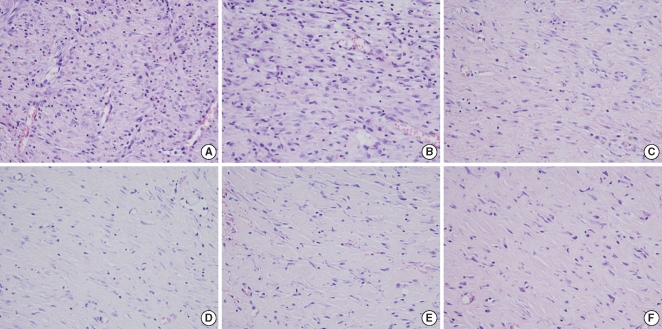
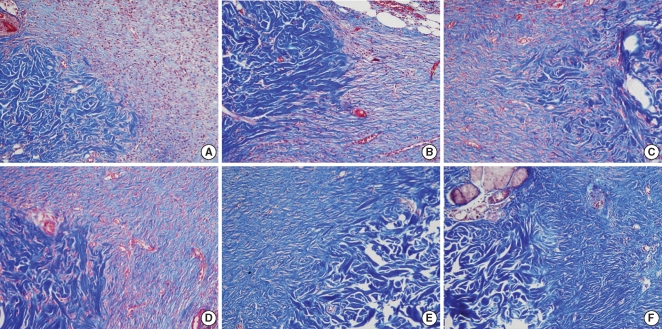
After surgery, 5 rats were sacrificed at the 2nd week, 5 rats at the 4th week and 5 rats at the 8th week. The skin wounds were harvested, including the epidermis, dermis and subcutaneous loose tissue with the surrounding normal tissue. The removed tissues were fixed in formalin, processed and then embedded in paraffin. The paraffin blocks were cut into 4-6 µm thick slices and the slices were stained with hematoxylin-eosin for histologic examination. To evaluate the degree of inflammation in both groups, the number of inflammatory cells, including the neutrophils, plasma cells and lymphocytes, was counted by a pathologist on high-power fields (×400) three times. The analysis of the degree of fibrosis was assessed by counting all the fibroblasts in 10 randomly selected high-power fields (×400). To evaluate the blood vessel proliferation, we counted the number of blood vessels on the high power field (×400), three times and we compared the mean number of blood vessels in each group. The thickness of the healing wound was determined by measuring the length from the basal layer of the epidermis to the deepest point of inflammation of the fibrosis under microscopic examination. We used Masson-Trichrome staining to evaluate the amount and density of the collagen. We scored the staining intensity as grade 1: the same or slightly decreased staining intensity compared with the surrounding normal tissue, grade 2: moderately decreased staining intensity compared with the surrounding normal tissue, and grade 3: markedly decreased staining intensity compared with the surrounding normal tissue.
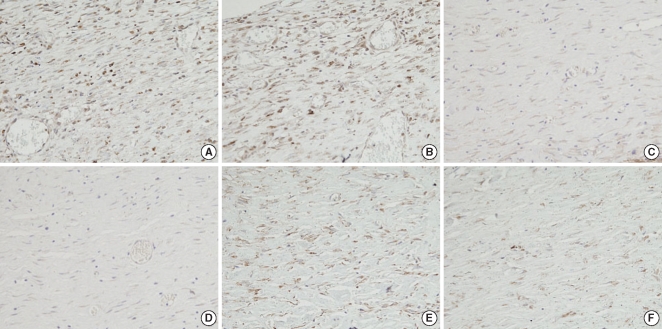
Immunohistochemical analysis was performed on the formalin-fixed, paraffin-embedded materials by using a primary antibody for TGF-β1 (diluted 1:30) (Santa Cruz Biotechnology Inc, CA, USA). Briefly, the sections were deparaffinized in xylene, rehydrated, washed in distilled water, immersed in 10 mM citrate buffer (pH 6) and then microwaved for 10 min. The sections were treated with a 3% H2O2 solution to reduce the endogenous peroxidase activity, and then they were washed in phosphate buffered saline; they were subsequently subjected to incubation with the primary antibody. Detection of the immunoreactive staining was obtained by the streptavidin biotin method with using an LSAB kit (DAKO, Carpinteria, CA, USA). The sections were subjected to a color reaction with diaminobenzidine and they were counterstained with Mayer's hematoxylin. TGF-β1 showed cytoplasmic staining as a brown color. The degree of the expression of the TGF-β1 in both wounds was assessed by counting the fibroblasts that showed a brown color in 10 randomly selected high-power fields (×400).
Statistical analysis
The Wilcoxon signed rank test was used to evaluate the difference of wound size between the Botox and control groups. The Mann-Whitney test was used to assess the differences of inflammation, fibroblasts, vessel proliferation, thickness of the wounds and the expression of TGF-β1 in the Botox and control wounds at the 2nd, 4th, and 8th week. McNemar's test was used to evaluate the difference of collagen maturation. All the P-values presented were two-sided, and the significance level was set at P<0.05.
RESULTS
Assessment of the wound size
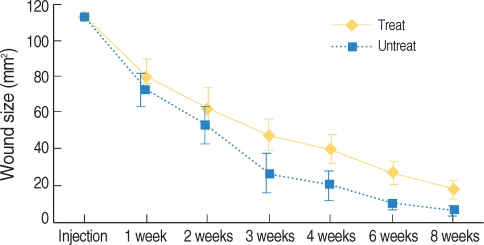
All the wounds were covered with the scab within the first day following the surgical procedure. The Botox and control wounds showed a progressive decrease in the size of the wound scab. There was no significant difference between the two groups for the formation and disappearance of the scabs. There were significant differences of wound size at the 3th (47.1±10.5 mm2 vs. 26.5±11.5 mm2, respectively) and 4th week (39.5±8.7 mm2 vs. 20.2±9.2 mm2, respectively) between the Botox and control groups (P<0.05) (Fig. 3, 4). At the 8th week after surgery, all the surgical wounds had the same appearance except for the size of the scars.
Histological assessment of wound healing
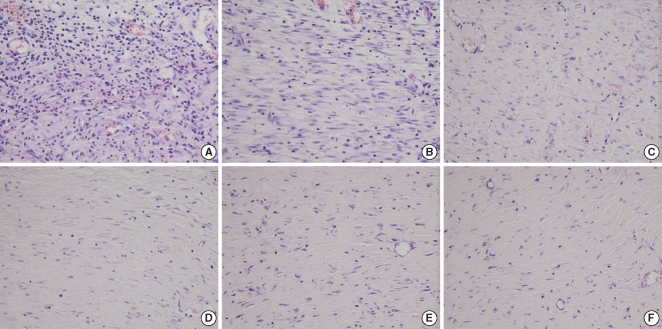
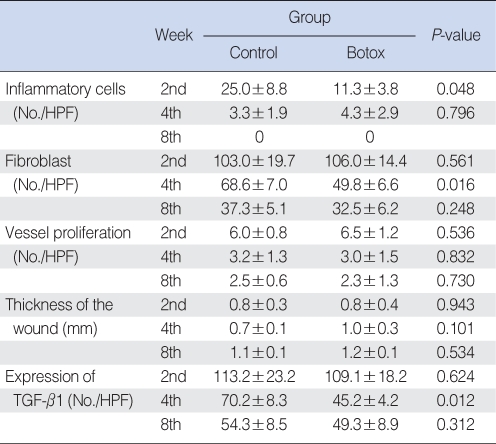
The Botox group (11.3±3.8, inflammatory cell number/high power field) showed less infiltration of inflammatory cells (11.3±3.8, inflammatory cell number/high power field) than that of the control group (25.0±8.8, inflammatory cell number/high power field) at the 2nd week after surgery (P<0.05) (Table 1, Fig. 5). However, there was no significant difference between the Botox and control groups for the degree of the infiltration of inflammatory cells at the 4th and 8th week after surgery.
The Botox group showed a lesser number of fibroblast and less fibrosis (49.8±6.6, number of fibroblasts/high power field) than that of the control group (68.6±7.0, number of fibroblasts/high power field) at the 4th week after surgery (P<0.05) (Table 1, Fig. 6). However, there was no significant difference between the Botox and control groups for the degree of fibrosis at the 2nd and 8th week after surgery (Table 1, Fig. 6).
There was no significant difference between the Botox and control groups for the degree of blood vessel proliferation, the thickness of the inflammatory lesion and the fibrosis at the 2nd, 4th, and 8th week after surgery (Table 1).
On the Masson-Trichrome stain for assessing the difference of the amount and density of collagen, there was no significant difference between the Botox and control groups for the amount of collagen and its density at the 2nd and 4th week after surgery. The Botox group showed a much stronger collagen density than that of the control group at the 8th week after surgery (P<0.05) (Table 2, Fig. 7).
On the immunohistochemical staining, there was a lower expression of transforming TGF-β1 in the Botox group (45.2±4.2, the number cells with a positive TGF-β1 expression/high power field) than that of the control group (70.2±8.3, the number of cells with a positive TGF-β1 expression/high power field) at the 4th week after surgery (Table 1, Fig. 8). However, there was no significant difference between the Botox and control groups for the expression of TGF-β1 at the 2nd and 8th week after surgery (Table 1).
DISCUSSION
Under normal conditions, the phases of wound healing are divided into three specific phases. The inflammatory phase is an attempt to limit the tissue damage by stopping the bleeding, sealing the surface of the wound and removing any necrotic tissue, foreign bodies or bacteria that are present. Inflammatory cells regulate the repair of the connective tissue matrix by the actions of various secreted cytokines. The proliferative phase is the repair processes of angiogenesis, fibroplasia and epithelization. This phase is characterized by the formation of granulation tissue, and this consists of a capillary bed, fibroblasts, macrophages and a loose arrangement of collagen, fibronectin, hyaluronic acid and bacteria. The maturation phase is the period of scar contracture with collagen cross-linking, shrinking and a loss of edema. At this phase, the acute and chronic inflammatory cells gradually diminish in number, angiogenesis ceases and fibroplasia ends. The equilibrium between collagen synthesis and collagen degradation is gradually restored. Although the three phases are described as a sequence of events, these phases may occur simultaneously, and the phases with their individual processes may overlap (17).
Tension is one of the important factors that determine the degree of fibrosis and scar formation. The relaxed skin tension lines reflect the tension created by underlying muscular contracture. Placement of scars in these lines or parallel to these lines will minimize the propensity to form hypertrophic scars. However, scars oriented against the relaxed skin tension lines are subject to repetitive tension and this causes scar hypertrophy.
Immobilization is a basic therapeutic principle for wound healing. Repeated microtrauma that's caused by continuous displacement of the healing tissue of the wound induces a prolonged and strong inflammatory response and an increased metabolic activity during the healing process. As a consequence, the extracellular deposition of collagen and glycosaminoglycans can intensify and lead to a hypertrophic scar.
Botulinum toxin type A acts at the neuromuscular junction by blocking vesicle transport of acetylcholine, and in essence, this produces a chemical denervation (13, 14). It has been used safely and effectively to control the symptoms of patients with disorders of a neural origin and voice disorders. In this study, it was observed that most surgical wounds injected with Botox had a larger size when compared with the control wound until the 8th week. There was a significant difference between the Botox and control groups for the wound size at the 3th and 4th week (P<0.05). The reason for the difference of wound size is presumed to be the decreased wound contracture power of the underlying muscle tension by Botox. Although there are possibilities that Botox has a direct effect on the inflammatory cells or it inhibits the mediators by a direct mechanism, we could not find any previous study that reported on these action mechanisms.
The wounds of the Botox group showed less infiltration of inflammatory cells than that of the control group at the 2nd week after surgery (P<0.05). However, there was no significant difference between the Botox and control groups for the degree of the infiltration of inflammatory cells at the 4th and 8th week after surgery. The reason for the difference of the inflammatory response at the 2nd week is presumed to be the decrease of microtrauma by the immobilization of the underlying muscle injected with Botox (Fig. 5).
For the histological findings on the H&E stain, the surgical wounds of the Botox group showed a significantly smaller number of fibroblasts and less fibrosis than the control wounds at the 4th week after surgery (P<0.05). The reason for the difference of the number of fibroblasts and the degree of fibrosis is presumed to be the decreased release of several cytokines, such as platelet-derived growth factor, TGF, epidermal growth factor, fibroblast growth factor and insulin-like growth factor, by the decreased inflammatory response and the short period of inflammation. This result suggests that Botox may be used to decrease the fibrosis during the healing process of surgical wounds by decreasing the underlying muscle tension (Fig. 9). However, there was no significant difference between the Botox and control groups for the thickness of the healing wound at the 2nd, 4th, and 8th week after surgery.
For the histological findings of Masson-Trichrome staining, the wounds of the Botox group showed a much greater amount of collagen than that of the collagen group at the 8th week after surgery. The collagen maturation in the Botox group was increased and the maturation was faster than that of the control group at the 8th week. The reason for the difference of the degree of collagen maturation is thought to be associated with the increased collagen maturation by the decreased inflammation response and the shortened period of the inflammatory phase for the surgical wounds of the Botox group (Fig. 9).
On the immunohistochemical staining, the wounds of the Botox group showed a lower expression of TGF-β1 than that of the control group at the 4th week after surgery. TGF-β is one of major important cytokines in the wound healing process. This cytokine is produced by a host of cells, including platelets, fibroblasts, smooth muscle cells, endothelial cells, keratinocytes, lymphocytes and macrophages (17). It increases collagen synthesis and stimulates keratinocyte migration, angiogenesis and fibroplasias (17). TGF-β1 is the most abundant isoform of TGF-β. The reason for the difference of the expression of TGF-β1 is the decreased inflammation response at the surgical wounds of the Botox group (Fig. 8).
For the surgical wound treated with mitomycin-C in a previous study, the histological analysis at the 1st month after treatment revealed a significant reduction in fibrosis of the wounds compared with the untreated wounds. After the third month, the degree of fibrosis was comparable between the surgical wounds treated with mitomycin-C and the surgical wounds that were not treated with mitomycin-C (18). In our study, although there was no significant difference between the Botox and control wounds for the number of fibroblasts at the 8th week after surgery, the wounds treated with Botox showed a smaller number of fibroblast and less fibrosis than did the control wounds at the 4th week after surgery. These results demonstrated that the antifibroblastic effect of Botox in surgical wounds was similar to that of mitomycin-C. Therefore, Botox can be used for the treatment and to prevent stenosis of tubular structures, such as the larynx, trachea and nasal cavity, the same as mitomycin-C. Further studies are needed that will focus on the relationship between the degree and the effective period of decreased fibrosis and the dose of Botox, and these studies will have to use an appropriate, large animal experimental model of airway injury.
Gassner et al. (19) have reported that an injection of Botox into the musculature underlying cutaneous wounds resulted in immobilization of the healing wounds and a significantly better cosmetic appearance than was seen for the nonimmobilized control wounds in primates. In the blinded, prospective and randomized clinical trial for forehead wounds, Gassner et al. (20) have also reported that botulinum toxin-induced immobilization of wounds enhances healing and they suggested its use in selected patients to improve the eventual appearance of the scar. Khera et al. (21) have reported the efficacy of Botox for preventing recurrence of uretheral stricture. The results of these above-mentioned studies are consistent with our results.
Wounds treated with mitomycin-C show delayed scab healing. It is suspected that mitomycin-C inhibits fibroblast proliferation and it might reduce the epithelial proliferation and healing. Yet in this current study, although Botox was shown to decrease the fibrosis in the healing process of surgical wounds, there was no significant difference between the Botox and control groups' surgical wounds at the period of the disappearance of scab. Although both the Botox and control surgical wounds were initially the same size, the surgical wounds treated with Botox became larger than the control wounds according to the decreased wound contracture and the reduced number of fibroblasts during the wound healing phase. These results suggest that Botox can be used to decrease the fibrosis and inhibit fibroblast proliferation without damaging the epithelial growth during the healing process of surgical wounds.
The Botox-treated group showed a larger wound size, less infiltration of inflammatory cells and less fibrosis, a much greater amount of collagen and a lower expression of TGF-β1 than that of the control group. Botox might be used, instead of mitomycin-C, to decrease the fibrosis of surgical wound without damaging the epithelial growth in situations for which decreased fibrosis is necessary, such as for treating laryngeal, tracheal and nasal stenosis. Further studies are needed that will determine the effective, safe doses of Botox injection and whether topical application of Botox cream might be as effective as injections of Botox. The expressions of the genes involved in wound healing in an appropriate laryngeal stenosis animal model should also be studied.