INTRODUCTION
Acoustic trauma results in the destruction of hair cells in the organ of Corti and nonsensory cells such as fibrocytes in the spiral limbus and spiral ligament [1]. However, the cellular and molecular mechanisms of acoustic trauma have not been fully elucidated. Recently, inflammation and the immune response have been recognized as important pathophysiological mechanisms involved in acoustic trauma [2-4]. Macrophages, the major innate immune cells, play a critical role in local cochlear immunity by contributing to inflammation and tissue repair [5]. Resident macrophages are present in the cochlea even before noise damage occurs, and they express CD45, F4/80, ionized calcium-binding adapter molecule 1, CD11b, and Fractalkine receptor (CX3CR1) [2,4,6,7]. These macrophages engulf cellular debris, produce proinflammatory cytokines, and present antigens [5,8]. Growing evidence suggests that infiltrating monocytes/macrophages are critical for acute inflammation and wound healing in other organs [9-12].
Intense acoustic overstimulation leads to a significant increase of immune cells in the cochlea. Several groups have reported that macrophage levels peak 3–7 days after acoustic trauma [6,13,14]. This increased population of macrophages is believed to result from the transformation of infiltrated monocytes, and not from the proliferation of resident macrophages [2,6]. However, there is a lack of research on infiltrated monocytes in the cochlea after noise exposure. In this study, we aimed to investigate the infiltration of monocytes and their transformation into macrophages using quantitative flow cytometry. CX3CR1–GFP reporter transgenic mice and the Ly6C antibody were used to visualize the macrophage lineage and inflammatory monocytes, respectively.
MATERIALS AND METHODS
Animals
Wild-type C57BL/6 mice and Cx3cr1GFP/GFP transgenic C57BL/6 mice were purchased from Jackson Laboratory (Maine) via Orient Bio (Seongnam, Korea). Cx3cr1+/GFP mice were used to visualize the resident macrophages. The mice were housed and maintained according to the animal research requirements, and all procedures were approved by the Institutional Animal Care and Use Committee (approval No. 2019-0182). Mice aged 4–12 weeks of both sexes were used. They were fed ad libitum and housed in cages in an environmentally controlled room under a 12-hour light cycle.
Noise generation
White noise (300–10,000 Hz) was generated using a personal computer and an amplifier (R-399; Inter M, Seoul, Korea) and delivered through speakers (290-8L; Altec Lansing, Oklahoma City, OK, USA) in a noise booth. Mice were continuously exposed to 120 dB peak equivalent sound pressure level (SPL) for 1 hour to induce permanent threshold shift and noise-induced cochlear inflammation.
Audiologic evaluation
The auditory brainstem response (ABR) was measured after anesthetizing the mice with xylazine (20 mg/kg intraperitoneal injection [IP]) and ketamine hydrochloride (30 mg/kg IP). The hearing level of each mouse was checked by measuring the ABR threshold with a Tucker-Davis Technologies (TDT) auditory evoked potential workstation (TDT, Alachua, FL, USA). Both ears of each mouse were stimulated with an ear probe sealed in the ear canal. The ABRs to click and tone stimuli were recorded, and thresholds were obtained for each ear. The ABRs were measured before and at 1 and 14 days after the noise exposure.
Histological preparation and immunostaining
After sacrificing the mice in a CO2 chamber, the bilateral temporal bones were dissected and fixed in 4% paraformaldehyde for 24 hours at 4°C after local perfusion with a fixative through the oval and round windows. After fixation, the samples were incubated in 1:3 EDTA (ethylenediaminetetraacetic acid) solution for 24 hours at 4°C for decalcification.
For obtaining a whole mount of the organ of Corti sample, decalcified cochleae were cut in half through the apex–oval window axis. Under optical microscopy, the cochleae’s lateral wall and bony capsule were carefully removed using forceps and microscissors. Other structures were trimmed while preserving the organ of Corti. All cochlear tissues were separated into apical, middle, and basal turns.
The tissues were blocked with 10% donkey serum and incubated with dye-conjugated Ly6C antibody (HK1.4, 128016; Biolegend, San Diego, CA, USA) and/or dye-conjugated F4/80 antibody (BM8, 123110, Biolegend) at 4°C overnight. The samples were then mounted with a mounting solution (Sigma-Aldrich, St. Louis, MO, USA) and viewed under an LSM780 confocal microscope (Zeiss, Jena, Germany). All immunostaining experiments were repeated in four biological replicates per time point.
Hair cell counts
After histological preparation, the decalcified cochlea was immunostained with fluorescent- tagged antibodies. FITC-conjugated phalloidin (1:200; P5282; Sigma-Aldrich) was used to stain cochlear hair cells and DAPI (1:5,000; Invitrogen, Carlsbad, CA, USA) for nuclear staining. Basal turns of the cochlea were examined under a confocal microscope (LSM700; Zeiss).
3D reconstruction imaging
Microscopy setting and clearing protocol was performed, as in our previous study [15]. Two-photon microscopy (LSM7MP; Carl Zeiss, Oberkochen, Germany) was used for generating imaging data. Zen software (Carl Zeiss) was used for image acquisition and basic image analysis. To create three-dimensional images and videos, IMARIS software (Bitplane, Zurich, Switzerland) was used. Sagittal plane images of 100 μm distance were stacked for the sagittal reconstructed image. Basal turn was carefully divided from decalcified cochlea, and mounted on a silicone bond in the petri dish, with basilar membrane positioned at the top. Distilled water was filled between the sample and water-immerse lens of the two-photon microcopy.
Flow cytometry
The cochleae were obtained from mice after cardiac perfusion with phosphate-buffered saline (PBS). Soft tissue was carefully removed from the cochlea in ice-cold PBS. After moving the cochlea to a new dish filled with ice-cold PBS, the bony capsule was partially removed with special care not to open the bone marrow. After the cochlea’s inner tissues were extracted through the opening, the remnants were discarded. The cochlear tissue was collected and trypsinized for 10 minutes at 37°C. The trypsinized tissue was ground and passed through a 40-μm filter. The dissociated cells were stained with anti-CD11b (M1/70, 101206, Biolegend), anti-Ly6G (1A8, 127608, Biolegend), and anti-Ly6C (HK1.4, 128016, Biolegend) antibodies (1:200) for 30 minutes. The stained samples were analyzed with a FACSverse II BD flow cytometer (BD Biosciences, Sparks, MD, USA). Each sample consisted of cells from two cochleae from a mouse, and five samples were included in each group.
Statistical analysis
For comparing multiple time points, a one-way analysis of variance test and post-hoc Tukey’s test were used. For comparing two independent groups with multiple time points, a two-way analysis of variance test and post-hoc Dunnett’s multiple comparison test were used. All graphs visualized the mean and standard error of the mean (error bar) values. IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA) and Prism 8.0 (GraphPad Software, San Diego, CA, USA) software were used for statistical analysis. A P-value <0.05 was regarded statistically significant.
RESULTS
Intense noise causes an ABR threshold shift and outer hair cell damage
We examined the ABR threshold shift and outer hair cell damage to study the effect of noise exposure on hair cells. Mice were continuously exposed to 120 dB peak equivalent SPL for 1 hour. Within 24 hours after noise exposure, the ABR thresholds of all frequencies were over 90 dB. Two weeks after noise exposure, there was no improvement in hearing thresholds across all examined frequencies (Fig. 1A), indicating that the noise level generated a permanent threshold shift. Next, whole-mount cochlear preparations were made 2 weeks after the noise exposure. Hair cells were stained with the myosin-VIIa antibody and Alexa Fluor 488-phalloidin, and observed under a confocal microscope. In the unexposed cochleae, all outer hair cells were preserved (Fig. 1B), whereas most of them were damaged in the basal section of the noise-exposed cochlea (Fig. 1C).
Infiltration of inflammatory monocytes
We visualized the monocytes infiltrating the cochlea after noise exposure by Ly6C immunofluorescence. Ly6C is a membrane marker of inflammatory monocytes [9,11,16,17]. In the spiral ligament, Ly6C-positive monocytes were easily identified on day 1 after noise exposure compared to days 3, 5, and 7 or the unexposed cochleae (Fig. 2).
Next, we confirmed the monocyte infiltration after noise exposure by flow cytometry, because it is challenging to quantify the clustered infiltrated monocytes on immunofluorescence images. A serial analysis by gating for CD11b+, Ly6G–, Ly6Chigh (indicators of inflammatory monocytes) showed a peak in the infiltration of inflammatory monocytes on day 1 post-exposure, followed by a subsequent decrease (Fig. 3) [17-21]. Leukocyte gating was performed with forward and side scatter signals, followed by gating for myeloid cells [22-24]. The CD11b antibody was used to gate the myeloid lineage immune cells, and the Ly6G antibody was used to exclude neutrophils because they also weakly express Ly6C [21]. The other cells in the CD11b+/Ly6G–/Ly6C– population consisted of dendritic cells, eosinophils, natural killer cells, and mostly macrophages [25]. Although there was no statistical significance, the absolute cell count of the CD11b+/Ly6G–/Ly6C– population gradually increased after noise, which may have resulted from the transformation of monocytes to macrophages, based on evidence from previous studies [22,25].
Macrophages migrate to the basilar membrane from the lateral wall
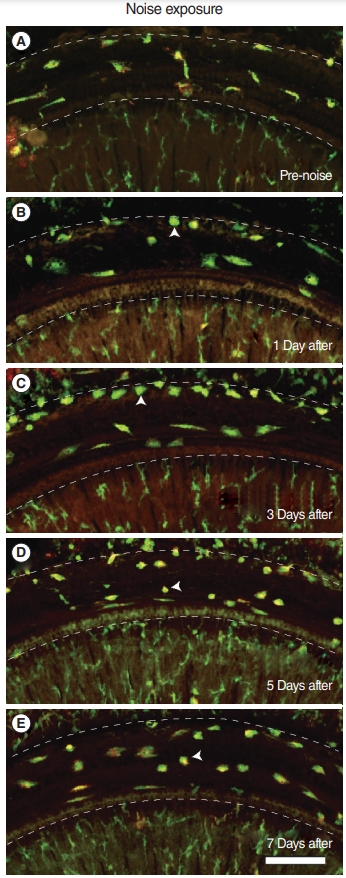
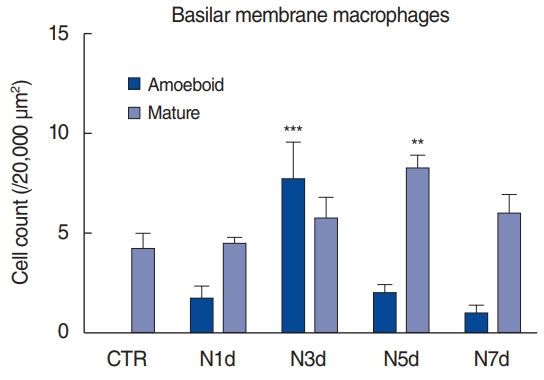
Based on whole-mount immunofluorescence performed on consecutive days after noise exposure, we investigated the migration of macrophages to the basilar membrane (Fig. 4, Supplementary Fig. 1). All the CX3CR1-positive (green) macrophages were costained with F4/80 antibody (red) throughout the entire field of vision. On day 1 after noise exposure, the cochlea showed no remarkable changes in the number and morphology of CX3CR1-positive macrophages displaying long and irregular shapes. However, on day 3 after noise exposure, the number of CX3CR1- positive macrophages increased, and they were lined along the crista basilaris (junction of the basilar membrane and lateral wall). These cells displayed round and amoeboid morphology, suggesting that they were newly infiltrated monocytes [4,5]. On days 5 and 7 after noise exposure, the CX3CR1-positive macrophages did not line the crista basilaris; instead, they had spread to the basilar membrane. The predominant morphology of macrophages at these time points was not amoeboid, but rather dendritic and irregular-shaped (Fig. 5).
DISCUSSION
Our findings revealed that the inflammatory monocytes migrated to the spiral ligament 1 day after noise exposure. In contrast, a change in the number of macrophages could be identified from day 3 onwards after noise exposure, based on immunofluorescence. The time taken for this population change suggests that the infiltrated monocytes transformed into macrophages. The crista basilaris is likely the major site of transformation since amoeboid macrophages lined this area 3 days after noise exposure. On days 5 and 7, the macrophages spread to the basilar membrane, potentially eliminating the degenerated cell debris and healing the damaged tissue.
Resident macrophages are present in the spiral ligament, spiral limbus, and spiral ganglion region, as well as beneath the basilar membrane, displaying various morphologies in different regions of the cochlea [3,6,26,27]. Previous studies have shown that while resident macrophages do not proliferate, circulating monocytes enter the cochlea and differentiate into mature macrophages after noise exposure. Hirose et al. [2] argued against macrophage proliferation in the spiral ligament based on immunohistochemical analyses after BrdU injections into mice following noise exposure. Okano et al. [6] also showed that transplanting GFP-labeled bone marrow into lethally irradiated mice resulted in an increased number of GFP+ macrophages in the cochlea. Consistent with these findings, we demonstrated that the cochlear macrophage population was likely increased by the migration of monocytes, which are macrophage precursors, from the vasculature—not by mitotic division of the resident macrophages.
Previous studies have shown that CX3CR1+ and CD45+ cells in the cochlea peak at 3 days post-noise exposure [2,6,7]. Yang et al. [4] showed an accumulation of cells displaying amoeboid morphology in the junction between the basilar membrane and lateral wall 4 days post-noise exposure. In line with these findings, we found several CX3CR1+ cells with amoeboid morphology in the crista basilaris 3 days after noise exposure, whereas they were not seen in the unexposed cochlea or 1 day after noise exposure. Instead, we found inflammatory monocytes 1 day after noise exposure. To the best of our knowledge, this is the earliest observation of immune cell infiltration after noise exposure. Our novel observations can explain the time course of the increase in the macrophage population after acoustic trauma, which has not previously been characterized. Monocytes from the blood started to infiltrate the cochlea parenchyma at 1 day after noise, and gradually decreased. They then began to transform into macrophages while losing the Ly6C phenotype at 3 days after noise exposure, which is consistent with the findings observed by other researchers, as described above.
Ly6C has been recently identified as an important marker of monocytes. Ly6Chigh monocytes display an inflammatory phenotype, while Ly6Clow monocytes are categorized as patrolling monocytes in the bloodstream [9,11,16,17]. We defined Ly6Chigh (CD11b+, Ly6G–, Ly6Chigh) cells as infiltrating monocytes based on previous studies [17-21]. In our study, Ly6Chigh monocytes accumulated in the lateral wall from the first day after noise exposure. These infiltrating monocytes transform into macrophages or dendritic cells in other organs [17,19,20]. Our findings, therefore, indicate that the cochlea has characteristics similar to other organs, allowing the transformation of monocytes into macrophages.
This study has some limitations. First, direct evidence regarding the transformation of monocytes into macrophages is still lacking. Therefore, future studies should track monocytes and gather meticulous FACS data. In the FACS analysis, the proportion of leukocytes in the total cell population was very low. The results may have been biased by contamination with non-leukocyte cells. Enrichment processes or additional cellular markers may be applied for more accurate analyses in future studies. Second, we focused on inflammatory monocytes, which are widely known to be involved in cochlear inflammation. However, other resident immune cells have also been reported in the cochlea after noise exposure [28]. The interaction of these cells with the monocytes that infiltrate following noise exposure needs to be evaluated.
In conclusion, we have demonstrated that acoustic overstimulation leads to the migration of circulating inflammatory monocytes into the lateral cochlear wall, which peaks 1 day after noise exposure. These infiltrated inflammatory monocytes acquire the properties of macrophages near the crista basilaris before spreading to the basilar membrane.
HIGHLIGHTS
▪ Noise exposure induced hair cell degeneration and immune cell recruitment.
▪ Monocytes infiltrated the spiral ligament 1 day after noise exposure.
▪ Macrophages in the crista basilaris increased in number on post-noise exposure day 3.
▪ Macrophages spread to the basilar membrane 5 days after noise exposure.