INTRODUCTION
Squamous cell carcinoma (SCC) of the oral cavity is typically seen after the fifth decade of life, and it is the sixth most common cancer in the world (1). Less than 4% of these lesions occur in patients younger than 40 years old (2). However, oral cavity cancers in younger patients have recently increased, and especially in female patients who do not consume alcohol or tobacco (3). Oral cavity cancers in younger patients have been suggested to show different clinical characteristics and disease progression from that seen in older patients (4). Son and Kapp (2), Amsterdam and Strawitz (5), and Sasaki et al. (6) concluded that the young patients have worse clinical outcomes than do the older patients. However, Friedlander et al. (7) and Pitman et al. (8) noted no significant difference in outcome between different age groups, and McGregor et al. (9), Clark et al. (10), and Hafkamp et al. (11) reported better outcomes in the younger age groups.
Thus, the previous studies have reported conflicting results about the clinical characteristics of oral cavity cancer in the young age groups, as well as having used different cutoff levels of age. In addition, there is a lack of reports that have compared between different age groups.
The purpose of this study was to analyze the recurrence and survival rates of SCC of the tongue in young patients who are under 45 years of age, and to compare them with older patients who have SCC of the tongue and who are over the age of 45 years.
MATERIALS AND METHODS
We retrospectively reviewed 105 patients who were treated for SCC of the tongue at our hospital between December 1994 and September 2008. Eighty-five patients were included who had not been treated before hospitalization for the same diagnosis, and they received their initial surgical treatment with a curative intent and a minimum follow up of 12 months. Twenty patients did not have sufficient data and they were excluded. Primary resection and neck dissection with an en bloc pattern was the surgical treatment for most patients in both groups. Selective neck dissection was done with a prophylactic intent for the clinically negative necks, and modified radical neck dissection was done for the clinically positive necks. In the limited cases of early cancer (<1 cm), primary resection was done without neck dissection. Adjuvant treatment was considered when the cancer free margin was insufficient (<5 mm) or when extracapsular invasion was found, or if multiple nodal metastases were found. Most patients were followed-up every 6 months for the first 2 years and then they were followed up every 12 months. The follow-up modalities were computed tomography (CT), magnetic resonance imaging (MRI) and a liver/bone scan or positron emission tomography (PET)/CT.
The study group was divided into two age groups with a cutoff level of 45 years. The following data was recorded from the patient files: age, gender, tobacco and alcohol consumption, staging, pathology, the treatment modalities, the length of the hospital stay and the clinical outcome. To evaluate the clinical outcome, we analyzed the recurrence rate, the site of recurrence and the overall and disease specific survival rates. The patients were staged according to the 2002 American Joint Committee on Cancer (AJCC) staging system. The institutional review board approved the retrospective review of the medical records.
Statistical analyses
To analyze the significance of the relationships between the two age groups and the clinical factors (such as gender, tobacco and alcohol consumption, TN stage, tumor cell differentiation, treatment modalities and the clinical outcomes), the chi-square test and Fisher's exact test were used, as appropriate. The recurrence rate was evaluated and compared using the Kaplan-Meier method and the log-rank test. The overall survival was determined and compared using the Kaplan-Meier method and the log-rank test. P-values <0.05 were deemed to indicate statistical significance. All the calculations were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
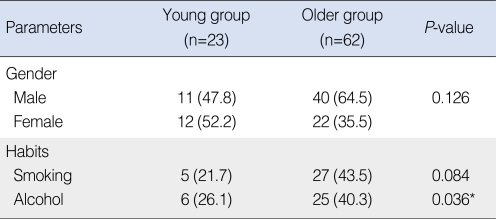
The young group consisted of 23 patients and the older group was comprised of 62 patients. The median age was 54.5 years (range, 23 to 86 years) with 51 men and 34 women. In the young age group (age <45 years), the median age was 38.0 years (range, 23 to 45 years) with 11 men and 12 women. In the older group (age Ōēź45), the median age was 62 years (range, 46 to 86 years), with 40 men and 22 women. The female:male ratio was not significantly different between the groups (P=0.126). Tobacco consumption was lower in the young group than that in the older group, but the difference was not significant (P=0.084). Alcohol consumption was significantly lower in the young group (P=0.036) (Table 1). The follow-up period ranged from 12 to 159 months (mean, 48 months) in the young group and 12 to 170 months (mean, 54 months ) in the older age group.
Stages and pathologic characteristics
Regarding the pathological stages, no significant difference existed between the groups in terms of the T or N stage (P=0.542 and P=0.308, respectively). Regarding the tumor cell differentiation, there were no significant differences between the groups (P=1.000).
Treatment modalities and clinical outcomes
The treatment modalities were recorded for all the patients: surgery alone was done in 17 patients (73.9%), surgery following adjuvant radiotherapy was done in zero patients (0%), surgery following adjuvant chemotherapy was done in one patient (4.3%), and surgery and adjuvant concurrent chemotherapy and radiotherapy were done in five patients (21.5%) in the young group; in the older group, surgery alone was done in 44 patients (71.0%), surgery following adjuvant radiotherapy was done in seven patients (11.3%), surgery following adjuvant chemotherapy was done in one patient (1.6%), and surgery and adjuvant concurrent chemotherapy and radiotherapy were done in ten patients (16.1%). No significant difference existed between the groups (P=0.273).
Regarding neck treatment, seven (30.4%) and 16 (69.6%) patients had bilateral neck dissection and unilateral neck dissection, respectively, in the young group, and 22 (35.5%) and 40 (64.5%) patients, respectively, did so in the older group. No significant difference existed between the groups (P=0.310).
Concerning surgical complications, one (4.3%) and two (8.7%) patients had flap failure and fistula, respectively, in the young group, and one (1.6%) and one (1.6%) patients had postoperative bleeding and wound infection, respectively, in the older group.
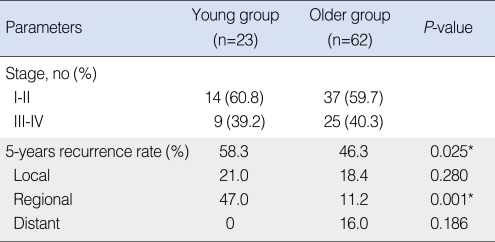
Twenty-seven patients experienced recurrences or metastases. In the young group, three (13.0%) cases were at the primary site, eight (34.8%) were in the neck and no case of distant metastasis was observed. In the older group, five (8.1%) cases were at the primary site, five (8.1%) cases were in the neck, and six (9.7%) cases had distant metastases. The 5 years-overall recurrence rate was 58.3% in the young group and 46.3% in the older group. The recurrence rate was significantly higher in the young group (P=0.025*). The 5 years-regional recurrence rate was 47.0% in the young group and 11.2% in the older group. The regional recurrence rate was significantly higher in the young group (P=0.001*) (Table 2).
Survival rate and the associated factors
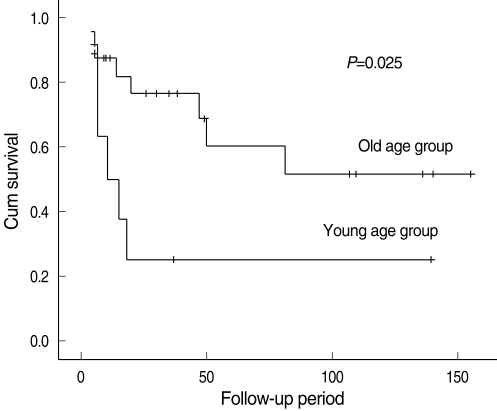
The final outcomes of the 85 patients were as follows: 18 patients died as a result of the disease, eight died from other causes and 59 survived with no further evidence of disease. The patients who were followed for more than 12 months had 5-years overall survival and disease-specific survival rates of 68.7% and 76.2%, respectively. The 5-years disease-specific survival rate was 65.6% in the young group and 71.0% in the older group; this difference was not significant (P=0.124) (Fig. 1).
Stage-matching analysis and the prognosis
Regarding the cancer staging, 14 (60.8%) patients were at an early stage (stage I, II) in the young group, and 37 (59.7%) patients were at an early stage in the older group. The young group had nine (39.2%) advanced-staged patients (stage III, IV), and the older group had 25 (40.3%) such patients. To compare the prognosis of the similarly staged patients between the groups, each age group was divided into the early- (stage I, II) and advanced-stage subgroups (stage III, IV), and these subgroups were compared.
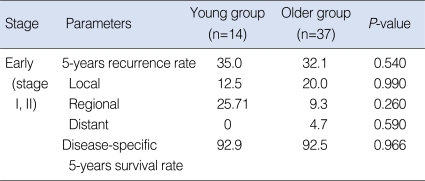
In the early-stage subgroup, nine (17.6%) patients experienced recurrences or metastases. In the young group, one (7.1%) case was at the primary site and two (14.3%) cases were in the neck, and there was no case of distant metastasis. In the older group, three (8.1%) cases were at the primary site, two (5.4%) were in the neck and one (2.7%) case had a distant metastasis. The 5 years-overall recurrence rate was 35.0% in the young group and 32.1% in the older group. No significant difference was observed between the groups (P=0.540) (Table 3). The 5-years disease-specific survival rate of the patients in the young group was 92.9%, and that in the older group was 92.5%. No significant difference in the disease-specific survival rate was observed between the groups (P=0.966) (Fig. 2).
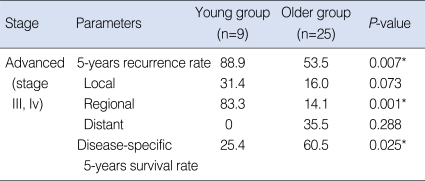
In the advanced-stage subgroup, 18 (52.9%) patients had recurrences or metastases. In the young group, two (22.2%) cases were at the primary site, six (66.7%) cases were in the neck and no case had a distant metastasis. In the older group, two (8.0%) cases were at the primary site, three (12.0%) were in the neck and five (20.0%) cases of distant metastases occurred. The 5 years-overall recurrence rate was 88.9% in the young group and 53.5% in the older group. The 5-years regional recurrence rate was 83.3% in the young group and 14.1% in the older group. The recurrence rate of the patients in the young group was significantly higher than that of the older group (P=0.007*), and especially for regional recurrence (P=0.001*) (Table 4). The 5-years disease-specific survival rate of the patients in the young group was 25.4%, whereas that in the older group was 60.5%. The disease-specific survival rate in the young group was significantly lower than that of the older group (P=0.025*) (Fig. 3).
DISCUSSION
SCC of the oral cavity is a rare disease in young people; fewer than 4% of these lesions occur in patients younger than 40 years of age (2). About 30% of the patients with SCC of the oral cavity have been reported to be younger than 45 years (12, 13).
Oral cavity cancers in young patients have recently increased, and especially in female patients who do not consume alcohol or tobacco. This disease has been suggested to show different clinical characteristics and disease progression from that in older patients (4). Atula et al. (14) in Finland found that the percentage of SCC of the tongue occurring in young adults had increased from 3% per year for the decade between 1953 to 1962 to 7% annually for the decade between 1983 to 1992. Lingen et al. (15) revealed that an increased p53 expression without a mutation in exon 5-9 was found in the SCC of the oral cavity in young, nonsmoking patients. Sorenson et al. (16) showed that the p53 mutation was less common in the tongue cancer of young patients with no history of alcohol or tobacco use.
We divided the patients who were diagnosed with SCC of the tongue into two age groups: over and under 45 years of age. We decided on this cutoff age based on the previous studies (2, 17). About 27% of the patients were under 45 years in our study group, and this was consistent with the previous studies (12-14).
Our findings were consistent with previous studies reporting that alcohol and tobacco use were less common in younger patients. No significant difference in smoking could be assessed due to the lack of data for some patients.
Friedlander et al. (7) and Pitman et al. (8) noted no significant difference in the outcome between different age groups. However, McGregor et al. (9), Clark et al. (10), and Hafkamp et al. (11) noted that the outcome of the younger group was better. Son and Kapp (2), Amsterdam and Strawitz (5), and Sasaki et al. (6) concluded that younger patients had worse clinical outcomes than did the older patients. Vargas et al. (18), in their study of 17 young females with invasive SCC of the tongue, they found that young women with SCC of the anterior tongue had significantly higher rates of recurrent disease and that the interval to recurrence was significantly shorter than that of older patients. The conflicting and inconsistent results of the previous reports seem to be due to the small numbers of patients in the young groups.
Our current report is partially consistent with the studies that found a worse prognosis in younger patients with tongue cancer. In the early-stage group, the recurrence rate was low and the survival rate was high for all ages. However, in the advanced-stage group, the recurrence rate was higher for the young patients, and especially for regional recurrence. In total, eight (88%) recurrent cases were seen in the young patients. There were six (66%) regional recurrences in the young patients, two of which were exclusively in the contralateral neck (level I, II). In the ipsilateral neck, there were four regional recurrences, and all of them were in level I. Regarding the possible cause of recurrence, two cases were treated by ipsilateral selective neck dissection with a prophylactic intent because of the clinical N0 stage. Subsequently, they were pathologic N2 stage and they recurred in the contralateral neck. Ipsilateral selective neck dissection is thought to be insufficient to eradicate neck disease. Three of the recurrent cases had multiple positive nodes pathologically (more than 13), and extracapsular invasion was found and they all recurred in level I. In one case, we found no obvious reason for recurrence. In two locally recurrent cases, the lack of a cancer-free margin was thought to be the reason for recurrence.
As noted previously, failure of regional control seemed to be the main cause of a poor prognosis in the young group. In the recurrent cases, the nodal disease was more aggressive than we had staged preoperatively. Thus, we think that more aggressive neck treatment may have prevented regional recurrence. However, a limitation of this study is its small sample size, and a larger multicenter study is needed.
According to our results, when the cancer is at an early stage, the outcome is similar at all ages, but as the cancer progresses, it transformed to a more aggressive form in the younger patients. This may have been due to different carcinogenesis at the gene level, so molecular studies would be useful.
In conclusion, tongue cancer at a young age has significantly different clinical outcome according to the tumor stage. Because advanced-stage tongue cancer leads to morbidity with a poor overall outcome and especially in young patients, it is important to detect it at an early stage. Regional recurrence seems to be main cause of the poor prognosis, and so we cautiously recommend that more complete neck treatment is needed.