Expression of EGFR and Microvessel Density in Middle Ear Cholesteatoma
Article information
Abstract
Objectives
Cholesteatoma destructs bony tissue by the interactions between hyperproliferative epithelial cells and subepithelial inflammatory cells. The aim of this study was to evaluate the expression of epidermal growth factor receptor (EGFR) and microvessel density (MVD) in middle ear cholesteatoma tissue in an effort to determine the relationship between expression of EGFR and neovascularization.
Methods
We evaluated the expression of EGFR and MVD by immunohistochemical staining for CD31 and Factor VIII in 32 cholesteatoma tissue samples and 7 normal postauricular skin samples. We also analyzed the correlation between EGFR expression and MVD.
Results
The expression of EGFR was higher in cholesteatoma than in postauricular skin, but the difference was not statistically significant. EGFR was more highly expressed in the suprabasal layer than in the basal layer. Using CD31 and Factor VIII, we analyzed the MVD and found that it was significantly higher in cholesteatoma than in postauricular skin, and significantly correlated with the expression of EGFR.
Conclusion
Our results suggest that overexpression of EGFR and neovascularization are correlated with the growth of cholesteatoma.
INTRODUCTION
Middle ear cholesteatoma is defined pathologically as the abnormal existence of keratinized squamous epithelium in the middle ear. This disease results in the destruction in the adjacent bony tissue as a result of the interaction between hyperproliferative epithelial cells and subepithelial inflammatory cells, but the mechanism regulating the growth of cholesteatomas is not clearly known. According to previous studies, the growth of middle ear cholesteatoma tissue is induced by various cytokines and growth factors secreted by inflammatory cells in the cholesteatoma matrix and subepithelial tissue (1). Moreover, it is thought that various cytokines from activated immunologic cells can lead to neovascularization, which can further contribute to the growth of a cholesteatoma.
Epidermal growth factor receptor (EGFR) is a 170 kDa transmembrane glycoprotein that is autophosphorylated by the binding of EGF. The subsequent biochemical reactions, such as phosphorylation of various target proteins, induce the growth of the cell (2). EGFR is known to be related to proliferation and differentiation of normal cells in vivo. The relationship between EGFR expression and proliferation of neoplastic cells has been suggested by studies showing that the cytoplasmic portion of EGFR has the same structure as the ν-erb-B transforming oncogene and that EGFR is overexpressed in several cancer cells (3). Overexpression of EGFR has also been reported in middle ear cholesteatoma, suggesting that is also associated with the proliferation of cholesteatomas.
It is well known that blood circulation is increased around the inflammatory tissue. Hyperproliferative tissue also requires an increase in blood supply. Cholesteatoma has both inflammatory and hyperproliferative properties. There are many studies about angiogenesis in inflammatory tissue and many studies have revealed that an increase in nutrients and oxygen supply plays an important role in the growth of neoplasms (4, 5). These facts suggest that the hyperproliferation of a cholesteatoma is related to neovascularization, and many studies on cholesteatoma have focused on angiogenesis and angiogenic factors (6, 7).
In this study, we evaluated the expression of EGFR and microvessel density (MVD) by immunohistochemical staining for CD31 and Factor VIII. Further, we characterized the relationship between the expression of EGFR and neovascularization in middle ear cholesteatoma tissue.
MATERIALS AND METHODS
We obtained cholesteatoma tissue from 32 subjects undergoing surgery as the study group, and normal postauricular skin tissue from 7 subjects as the control group.
Immunohistochemical staining
Immunohistochemical staining was performed to evaluate the expression of EGFR and MVD. The tissues were fixed in 10% buffered neutral formalin solution and embedded in paraffin. For immunohistochemical staining, 4 µm-paraffin embedded tissue sections were prepared on poly-L-lysine-coated slide (Sigma, St. Louis, MO, USA). The tissue sections were deparaffinized twice in 100% xylene for 10 minutes, and then treated with 100%, 90%, 70%, and 30% ethyl alcohol for 10, 5, 5, and 5 minutes, respectively, prior to rehydration with distilled water. The slides were then treated with 3% H2O2 in methanol for 10 minutes and washed with distilled water for 5 minutes to quench endogenous peroxidase activity. Antigen retrieval was performed by heating the slides for 5 minutes in citrate buffer (pH 6.0) in a staining jar and by cooling for 20 minutes. Then, sections were washed twice with Tris-buffered saline (TBS, pH 7.6) for 5 minutes and left with blocking antibody at room temperature for 20 minutes to remove nonspecific binding. After that, sections were incubated for 1 hour with 1:30 dilution of monoclonal antibody to EGFR, CD31 and Factor VIII (Novocastra, Newcastle, UK) in a wet chamber. After washing the sections three times with TBS for 5 minutes, we incubated the sections for 20 minutes at 37℃ with the secondary antibody, a biotinylated goat-anti mouse IgG (DAKO, Carpinteria, CA, USA), and then for an additional 20 minutes with streptovidin-peroxidase conjugate (DAKO). After washing with TBS, 3,3'-diaminobenzidine tetrahydrochloride (DAB) was used to visualize the peroxidase activity and Mayer's hematoxylin was used to counterstain. The sections were mounted with Canada balsam.
Quantitative analysis of EGFR expression
All specimens were independently analyzed by two pathologists who were blinded to the specimen information. Both the basal and suprabasal layers of the epitheliums of normal postauricular skin and cholesteatoma were analyzed for positive staining.
The EGFR positive-staining rates were determined in the areas where the full thickness of the epidermis could be clearly seen, and we designated cells that stained brown in the cytoplasm or membrane as positive. The results for the specimen training were recorded as negative (-) if the percentage of positive cells was under 10%, weakly positive (+) if 11-30%, moderately positive (++) if 31-60%, and strongly positive if over 60%. After the identification of well-stained areas under a light microscope at 100-fold magnification, we calculated the average percentage of three different spots at 400-fold magnification.
Calculation of MVD by immunohistochemical staining of CD31 and Factor VIII
In the areas of brown staining for microvessels (venule and capillaries) observed under a light microscope at 100-fold magnification, we calculated the average numbers of microvessels in three different areas at 200-fold magnification.
Statistical analysis
Fisher's exact test, Mann-Whitney U-test and Spearman correlation test were applied for the statistical analysis using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) program, and a P-value<0.05 was defined as a statistically significant difference.
RESULTS
Expression of EGFR
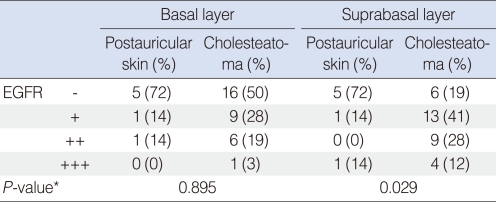
Among 7 normal postauricular skin samples, there was no detectable expression of EGFR in 5 samples. In cholesteatoma, EGFR staining was weakly positive in 11 samples, moderately positive in 9 samples, strongly positive in 4 samples, and not detected in 8 samples (Fig. 1, Table 1).
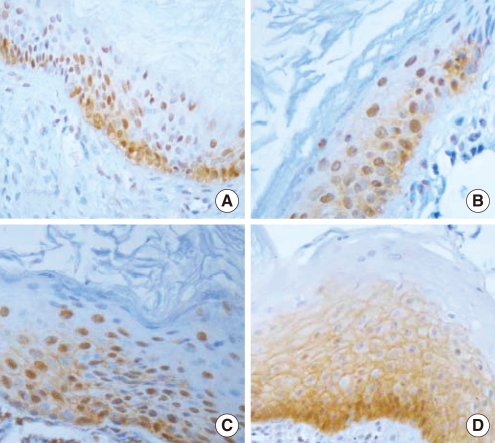
Immunohistochemical demonstration of epidermal growth factor receptor in cholesteatoma (×400). Negative (A), weakly positive (B), moderately positive (C) and strongly positive (D) findings.

Comparison of epidermal growth factor receptor (EGFR) expression in cholesteatoma and postauricular skin
We analyzed the expression of EGFR by separating the epithelium into suprabasal and basal layers. In the basal layer, 5 negative, 1 weakly positive, 1 moderately positive and 0 strongly positive cases were detected in the postauricular skin, and 16 negative, 9 weakly positive, 5 moderately positive and 2 strongly positive cases were found in the cholesteatoma group. The two groups were composed mostly of either negative or weakly positive cases, and there were no significant difference between the two groups (P=0.895).
In the suprabasal layer, 5 negative cases, 1 weakly positive case, 0 moderately positive cases and 1 strongly positive case were detected in the postauricular skin, and 6 negative, 13 weakly positive, 9 moderately positive and 4 strongly positive cases were detected in the cholesteatoma samples. Most of the postauricular skin group stained negatively, but cholesteatoma group had more moderately and strongly positive cases, and these differences in staining were statistically different between the two groups (P=0.029) (Table 2).
MVD
The MVD was analyzed using CD 31 staining in the two groups. We found 14.14±4.34 in postauricular skin and 27.63±13.21 in cholesteatoma (P=0.004) (Fig. 2). Using Factor VIII staining, MVD was 8.71±4.03 in the postauricular samples and 18.97±8.87 in the cholesteatoma samples (P=0.002) (Fig. 3). Thus, the MVD was significantly higher in the cholesteatoma samples, regardless of the staining method used (Table 3).
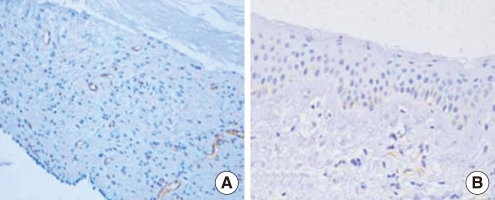
Immunohistochemical demonstration of microvessel density using anti-CD31 antibody in a cholesteatoma (A) and postauricular skin (B) (×200). Cholesteatoma tissue shows high microvessel density, but postauricular skin shows low microvessel density.

Immunohistochemical demonstration of microvessel density using anti-Factor VIII antibody in a cholesteatoma (A) and postauricular skin (B) (×200). Cholesteatoma tissue shows high microvessel density, but postauricular skin shows low microvessel density.
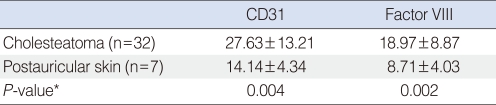
Microvessel density by immunohistochemical staining of CD31 and Factor VIII in cholesteatoma and postauricular skin
The correlation between CD31 and Factor VIII staining was statistically significant (coefficient of correlation, 0.814; P<0.001) (Fig. 4).
Correlation between the expression of EGFR and MVD
For the analysis of a correlation between the expression of EGFR and MVD, we divided the expression of EGFR into two groups, the weakly expressed group (negative or weak positive) and the highly expressed group (moderate to strong positive). MVD using CD31 was 20.37±10.82 in the weakly expressed group and 38.23±8.37 in the highly expressed group, which is a statistically significant difference (P<0.001). MVD using Factor VIII was 15.00±7.00 in the weakly expressed group and 24.77±8.26 in the highly expressed group, representing a statistically significant difference (P=0.002) (Table 4).
DISCUSSION
EGFR is a 170 kDa transmembrane glycoprotein composed of an intracellular tyrosine kinase domain, extracellular ligand-binding domain and transmembrane domain. The binding of EGF to the extracellular domain induces the activation of tyrosine kinase, which initiates a signal transmission pathway that induces cell proliferation by expression of transcription factors like c-jun and c-fos in the nucleus (8). Also, the structural non-discrimination between the cytoplasmic portion of EGFR and ν-erb-B transforming oncogene is evidence of an important role for EGFR in regulation of growth in both normal and abnormal cells (9). EGFR is normally expressed in all kinds of cells from the germinal layer, and it is expressed highly in gastrointestinal tract and the urogenital tract. The overexpression of EGFR has been reported for various diseases such as psoriasis, and in hyperproliferating such as skin cancer (10), brain cancer (11) and head and neck cancer (12). Also, it has been reported that EGFR is overexpressed in cholesteatoma. Some studies have reported the overexpression of EGFR in both the basal and suprabasal layer (13), but other studies have reported overexpression of EGFR in only the suprabasal layer (14).
In this study, the overall expression of EGFR is higher in cholesteatoma than in postauricular skin, but the difference in expression is not statistically significant. In our analysis in which the epithelium was separated into a basal and suprabasal layer, cholesteatoma showed higher EGFR expression than postauricular skin in the suprabasal layer. Thus, it is thought that hyperproliferation is a characteristic of cholesteatomas.
Many studies have reported on the importance of the interaction between the epithelium and connective tissue (the dermis of normal skin and matrix of cholesteatoma) in proliferation and differentiation. In cholesteatomas, the interaction between the epithelium and matrix produces various cytokines and growth factors, which induce proliferation, differentiation, and neovascularization of the cholesteatoma (15, 16). Many studies have reported the neovascularization in the cholesteatoma, and have uncovered the correlation between cholesteatoma and the expression of angiogenic factors such as β-fibroblast growth factor (FGF), FGF-2 and vascular endothelial growth factor (VEGF). Sudhoff et al. (7) reported that the expression of angiogenic factors such as β-FGF, TGF (transforming growth factor)-α, TGF-β1 and VEGF in cholesteatoma was 4.3 fold higher than in the middle ear mucosa and twice more than in the external auditory canal skin. They also detected more neovascularization in more severely inflamed tissue in cholesteatoma, so they concluded that neovascularization could have an important role in inflammation and inflammatory reactions in cholesteatoma. Stammberger et al. (17) conducted a morphological analysis of the newly developed vessels in cholesteatoma using Factor VIII, and revealed more neovascularization than in the dermis of normal skin. Hyperproliferative tissue like middle ear cholesteatoma requires more vascularization, and the neovascularization is thought to contribute to the migration of the epithelium of cholesteatoma into the middle ear. So, it has been suggested that the matrix of cholesteatoma can invade the middle ear by this composite action.
For the morphological observation of the MVD, the surface antigens of the vascular endothelium such as CD34, CD31 and Factor VIII were used. This study used Factor VIII and CD31. In general, CD31 reacts to the endothelial cells of the blood vessel, and Factor VIII reacts to the endothelial cells of the blood and lymphatic vessels (18). A cholesteatoma also has lymphatic tissue in the matrix and surrounding tissues, and initially this study intended to identify MVD and microlymphatics density using both CD31 and Factor VIII. It is assumed that subtraction of MVD using CD31 from the microvessel plus microlymphatics densities using Factor VIII in the same section could reveal the microlymphatics density. But our results showed less density in Factor VIII staining than in CD31 staining. We thought that Factor VIII was less expressed in cholesteatoma tissue than CD31. Although the correlation between the Factor VIII and CD31 suggested that these factors would be useful in the identification of MVD, Factor VIII is less expressed in cholesteatoma tissue, so it is thought that CD31 is more useful. This study revealed statistically higher MVD than in postauricular skin, and this result corresponds to the results of the study of Stammberger et al. (17) or Anniko and Mendel. (19).
Up until now, there have only been a few studies directly demonstrating the correlation between growth factor and neovascularization in cholesteatoma. Stammberger et al. (20) reported a significant correlation between growth factor TGF-α and MVD using Factor VIII, and Sudhoff et al. (21) reported that the growth of the epithelium and vascular endothelium was increased with the inflammatory reaction of the cholesteatoma. In this study, the more the expression of EGFR increased, the higher the MVD was in both basal and suprabasal layers of the cholesteatoma.
In conclusion, MVD using CD31 and Factor VIII was significantly higher in a cholesteatoma than in postauricular skin, and the MVD was significantly correlated with the expression of EGFR. The overexpression of EGFR in the middle ear cholesteatoma might be closely related to the neovascularization, and it is thought that overexpression of EGFR and neovascularization are correlated with the growth of a cholesteatoma.
Notes
No potential conflict of interest relevant to this article was reported.