The Effect of Nano-Silver on Allergic Rhinitis Model in Mice
Article information
Abstract
Objectives
Silver has long been known as a strong antimicrobial and disinfectant. Several types of nano-silver coated products have been developed. However, the antimicrobial and disinfectant characteristics of nano-silver have not been well studied. The aim of this study was to investigate the effect of nano-silver on allergic inflammation in a mouse model.
Methods
Female BALB/C mice were sensitized by intraperitoneal injection of ovalbumin (OVA) and aluminium hydroxide on days 0, 7, 14, and 21. Mice were challenged with intranasal instillation of OVA. Nano-silver was also administered nasally prior to intranasal instillation of OVA. Severity of allergic rhinitis was assessed according to nasal symptoms, serum OVA-specific IgE level, interleukin (IL)-4, IL-10, and interferon (INF)-γ levels in nasal lavage fluid. Hematoxylin-eosin stain and periodic acid-Schiff stain were performed for evaluation of histological change.
Results
Nano-silver attenuated manifestation of nasal symptoms in sensitized mice and inhibited production of OVA-specific IgE, IL-4, and IL-10, however, it had no effect on INF-γ level. In addition, the degree of inflammatory cell infiltration and goblet cell hyperplasia was attenuated by nano-silver.
Conclusion
These results suggest that nano-silver may effectively reduce allergic inflammation in a mouse model of allergic rhinitis. Through its properties as an anti-inflammatory agent, nano-silver may be a useful therapeutic strategy.
INTRODUCTION
Silver vessels were used in ancient times for preservation of water and wine, and silver powder was believed to have beneficial healing and anti-disease properties. Disinfectant properties of silver for hygienic and medicinal purposes are time honored and prominent, however, the mechanism is not yet fully understood. It exhibits broad-spectrum antimicrobial activity in vitro by binding to microbial DNA, which prevents bacterial replication and binding to the sulfhydryl groups of the metabolic enzymes in the bacterial electron transport chain, causing their inactivation [1]. With the use of nanotechnology (NT), nano-silver particles with antimicrobial and disinfectant properties have been developed. However, some materials do exhibit toxicity to mammalian cells, even if they are biochemically inert and biocompatible in size [2,3]. For clinical use in a medical setting, agents should be safe and available. The nano-silver (Medisil; NEXtec Co., Daegu, Korea) used in this study was stabilized with a polymer capsule that can dissolve and the nanoparticles are then released to react with contact cells as a catalyst. High concentrations of nano-silver have been found to be cytotoxic to peripheral blood mononuclear cells (PBMCs); however, at safe concentrations, it can alter cytokine production in PBMCs [4].
Nano-silver is used for wound management, particularly for treatment of burns, and in urethral and central line catheters to prevent growth of slime-containing biofilms that promote bacterial infection and sepsis [5]. Although several types of silver coated prosthesis have been developed, their ability to prevent infection has not been collectively addressed. Antimicrobial and disinfectant characteristics have not been well studied and no standardized method has been developed for determination of these characteristics. Many animal models for the study of allergic rhinitis have been reported and murine models are especially useful for study of the immunologic mechanism of this disease [6,7]. Nano-silver is well known for its anti-bacterial, anti-viral, and anti-fungal properties, however, the anti-inflammatory effects of nano-silver have not been well studied. In this study, we used a mouse model of allergic rhinitis for evaluation of the effect of nano-silver instillation on nasal mucosal inflammation and allergic symptoms.
MATERIALS AND METHODS
Preparation of nano-silver
The nano-silver colloidal solution (Medisil) at a concentration of 5,000 ppm was prepared by chemical reduction of silver ions by physical methods, with reducing agents and stabilizers. First, 31.5 g of silver nitrate was dissolved in 3.7 L of distilled water, followed by addition of 40 g of stabilizer. Second, the reducing agent was dissolved in distilled water and this solution was dropped slowly into the silver ion-stabilizer solution under sonication. After dropping the solution, another stabilizer was dissolved and stirred vigorously for 1 hour. The stabilizer included sodium hydroxide, which neutralized the nano-silver, the final products were sodium nitrate and silver. The particle size of the nano-silver and UV-visible spectrum of the nano-silver colloidal solutions was characterized by transmission electron microscopy (TEM) and a size analyzer (ELS-8000; Otsuka electronics, Osaka, Japan). TEM images of the nano-silver revealed an average size of approximately 1.5 nm, with a size distribution ranging from 1 to 2.5 nm. In addition, results of size distribution analysis using the size analyzer showed that the distribution of the nano-silver particles ranged from 1 to 2.5 nm with an average size of 1.3 nm. The particles were well dispersed in the colloidal solution.
Animals and experimental protocol
Female BALB/c mice, which were six-week-old and free of murine specific pathogens, were obtained from Hyosung Science Inc. (Daegu, Korea). They were maintained under standard laboratory conditions in a pathogen-free cage. Food and water were freely available and all animal experiments in this study were conducted in accordance with the guidelines of the Institutional Review Board of Daegu Catholic University Medical Center.
Mice were sensitized by administration of an intraperitoneal injection of OVA (75 µg) in 200 µL of phosphate buffer solution (PBS) containing 2 mg of aluminum hydroxide (Sigma Aldrich, St. Louis, MO, USA) in a total volume of 200 µL on days 0, 7, 14, and 21. On days 22-30 after initial sensitization, mice were challenged with nasal instillation of OVA 500 µg in 20 µL of PBS into bilateral nasal cavities. The control group was challenged with PBS instead of OVA.
Nano-silvers were dissolved in PBS and administered by micropipette (20 µL at 10, 1, and 0.1 ppm), beginning 1 hour before each challenge on days 22-30. The control group was treated with PBS.
Evaluation of nasal symptoms
The number of sneezing and nose rubbing motions during a period of 15 minutes after the final allergen challenge was recorded and compared with that of the control group.
Nasal lavage fluid collection
Nasal lavage fluid (NLF) was collected 24 hours after the last intranasal provocation with OVA. Nasal lavage by an 18-gauge catheter was performed after partial tracheal resection. The catheter was inserted into the tracheal opening in the direction of the upper airway and into the nasopharynx. Nasal passages were gently perfused with 1 mL cold PBS and collected in a tube. NLF was centrifuged at 2,000 rpm for 7 minutes at 4℃, and the supernatant was stored at -70℃. Amounts of interleukin (IL)-4, IL-10, and interferon (INF)-γ in NLF were measured using an ELISA quantitation kit (R&D Systems, Minneapolis, MN, USA).
Measurement of OVA-specific IgE in serum
Blood specimens were collected from the inferior vena cava 24 hours after the last intranasal provocation. Serum was obtained by centrifugation and stored at -70℃. OVA-specific IgE level in serum was measured using the ELISA kit (Pharmingen, San Diego, CA, USA).
Histologic evaluation of nasal mucosa
Mice were painlessly sacrificed with a lethal dose of intraperitoneally administered pentobarbital sodium (120 mg/kg) 24 hours after the last intranasal provocation. Their decapitated heads were immersed in 10% neutral formalin overnight. The heads were then stripped of the eyes, skin, muscle, and the mandibles were excised. Specimens were decalcified until they were soft in 0.25 mol/L ethylenediaminetetraacetic acid for 24 hours. The heads were trimmed with a fresh razor blade, with excision of the anterior portion of the nose and brain, leaving a portion of the nasal sinus, which measured approximately 8 mm in length from anterior to posterior. The resulting blocks were embedded in paraffin and sectioned anterior to posterior at 5-µm thickness.
Three anatomically similar sections were chosen from each mouse for analysis. The first section, the most anterior, was at the level of the maxillary sinuses. The second section, more posterior, was at the end of the maxillary sinuses and the beginning of the complex ethmoid turbinals. The third section, most posterior, contained the brain superiorly.
Appearance of inflammatory cell infiltration and epithelial thickness was quantified in hematoxylin and eosin stained sections at ×200 and ×400 magnification. Goblet cell numbers were quantified in periodic acid-Schiff (PAS) stain at ×200 magnification. All tissue sections were examined blindly with respect to the source of the tissue and counts were determined at three different mucosal areas for each of the three sections per mouse.
The presence or absence of submucosal inflammatory cell infiltration was quantified into four categories: 0, no; 1, mild, occasional scattered inflammatory cells; 2, moderate; 3, severe, diffuse infiltration of inflammatory cells. Epithelial thickness was directly measured on a scale of ×400 magnification and average number of goblet cells was counted at four different areas per 1 mm2 of nasal mucosa by eyepiece reticule.
Statistical analysis
All measured parameters were expressed as means±SD. The Mann-Whitney U-test (SPSS Inc., Chicago, IL, USA) was performed for statistical analysis of data. A probability value less than 0.05 was considered statistically significant.
RESULTS
Allergic behavior
The mean behavior score, which was the total number of sneezing and nasal rubbing motions during a period of 15 minutes after the final challenge, was 7.6±2.1 in the control group, 32.1±4.3 in the allergic rhinitis (AR) group, 24.8±2.8 in the 0.1 ppm group, 22.6±2.7 in the 1 ppm group, and 15.5±1.6 in the 10 ppm group. Behavior scores were elevated in the AR group and were lower in the 1 and 10 ppm groups, compared with the AR group (P<0.05). Other comparisons among the three nano-silver treated groups failed to show significance.
NLF cytokine and serum OVA-specific IgE
Repeated intranasal administration of nano-silver resulted in a significant decrease in serum OVA specific IgE antibody level (P<0.05) (Fig. 1). Other comparisons among the three nano-silver treated groups failed to show significance.

Ovalbumin (OVA)-specific IgE levels in serum and cytokine levels in nasal lavage fluid of each study group. (A) Serum IgE, (B) interleukin (IL)-4, (C) IL-10, and (D) interferon (INF)-γ. OVA-specific IgE antibody, IL-4, and IL-10 production was significantly inhibited by nano-silver. NC, negative control group; AR, allergic rhinitis group. *P<0.05 vs. the AR group. †P<0.05 vs. the NC group.
IL-4 and IL-10 levels in NLF showed a significant increase in the AR group (9.1±2.4 and 9.9±3.2 pg/mL), compared with the control group (4.8±0.9 and 2.9±0.7 pg/mL). IL-4 and IL-10 production were significantly inhibited by nano-silver (P<0.05). INF-γ in NLF did not differ significantly among the AR group, control group, and nano-silver treated group (Fig. 1).
Histologic changes
Whereas no or minimal inflammation was noted in the control group, all experimental group showed an increase in inflammatory cell infiltration of the submucosal area. Most of the inflammatory cells were lymphocytes and some of them were eosinophils. The degree of inflammatory cell infiltrations showed an increase in the AR group (1.8±0.6). Nano-silver induced significant inhibition of inflammatory cell infiltration (1.2±0.4 with 0.1 ppm, 0.9±0.6 with 1 ppm, and 1.0±0.7 with 10 ppm; P<0.05).
PAS-positive goblet cells in nasal mucosa showed a significant increase in the AR group (37.1±14.3), compared with the control group (1.4±1.2), nano-silver 1 ppm (8.2±6.1) and 10 ppm (8.1±5.6; P<0.05) (Fig. 2). However, nano-silver 0.1 ppm did not have a significant influence on PAS-positive cells (20.8±10.3) (Fig. 3).
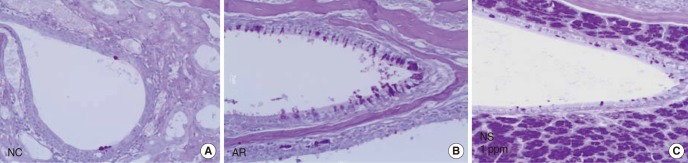
Photographs of periodic acid-Schiff (PAS) stain of nasal mucosa. The number of PAS positive cells was increased in the allergic rhinitis (AR) group compared with the negative control (NC) group. The number of PAS positive cells was decreased in the nano-silver (NS) treated group, compared with the AR group.
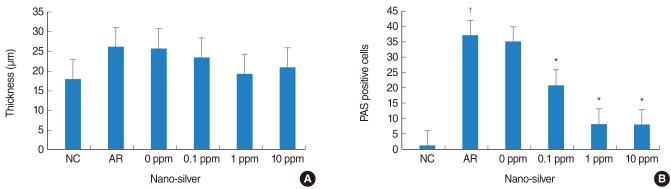
Epithelial thickness and the number of periodic acid-Schiff (PAS) positive cells of nasal mucosa tissue of each study group. (A) Epithelial cell thickness was not influenced by nano-silver treatment, (B) the number of PAS positive cells was decreased in the nano-silver treated group, compared with the allergic rhinitis (AR) group. NC, negative control group. *P<0.05 vs. the AR group. †P<0.05 vs. the NC group.
Thickness of epithelial cells in nasal mucosa showed a significant increase in the AR group (26.2±7.1 µm) compared with the control group (17.1±2.8 µm). However, nano-silver did not have a significant influence on the thickness of epithelial cells (Fig. 3).
DISCUSSION
Allergic rhinitis is the most common chronic condition in both adult and pediatric populations, affecting 10% to 30% of adult and 20% to 40% of children [8]. Presentation of an allergen to lymphocytes leads to release of Th2 cytokines, like IL-4, IL-5, and IL-13, which promote IgE production and the infiltration of the mucosa by inflammatory cells. Several promising pharmacological and immunological treatments for AR are available and these include antihistamines, corticosteroids, decongestants, mast cell stabilizers, leukotriene modifiers, anti-IgE antibodies, phosphodiesterase inhibitors, intranasal heparin, and immunotherapy. All of these have been shown to be effective and safe in clinical trials, in addition new and promising pharmacological and immunological approaches will certainly change the pattern of treatment of allergic rhinitis. In this in vivo study, a new chemical agent, nano-silver shows the anti-allergic effect.
Nano-silver developed through nanotechnology exhibits remarkably unusual physicochemical properties and biological activities. Remarkably strong anti-microbial and anti-inflammatory activity is a major direction for development of nano-silver products. Nanoparticles can undergo a series of processes, including binding and reacting with proteins, phagocytosis, deposition, clearance, and translocation. At the same time, nanoparticles can elicit a spectrum of tissue responses, such as cell activation, generation of reactive oxygen species, inflammation, and cell death [9,10]. Controversy remains with regard to the beneficial and harmful effects of nano-silver. These conflicting results may be attributable to several confounding factors, such as particle size, surface charge, delivery method, route of administration, duration of administration, and dosage.
Nanoparticles, less than 0.1 µm in mass median aerodynamic diameter, have been shown to be increased in ambient air and are postulated to affect the cardiopulmonary system [11,12]. Physical characteristics of nanomaterials, such as size, shape, and surface properties, exert a toxic effect that goes beyond that associated with their chemical composition [13]. Silver nanoparticles have been reported to exert considerable toxicity in multicellular nematodes through a dramatic decrease in their reproduction potential [14]. Although nano-silvers have toxic effects, nano-silver products are currently on the market throughout the world as medical or personal products. There are currently no standardized methods for production of nano-silver, a task made more difficult by the wide variation in clinical silver product delivery systems and silver formulations [15]. Nano-silver used in this study was stabilized with a polymer capsule, such as the surfactant of cleanser. When capsulated nano-silver comes in contact with cells, a polymer capsule can be collapsed and nanoparticles react with contact cells as catalysts without intracellular accumulation. They can also dissolve in water without aggregation. These characteristics make them suitable for in vivo studies and intranasal application. Intranasal application also prohibits the systemic absorption and toxic effects of nano-silver. However, in terms of clinical usage, agents should be safe and available. Our previous study showed that nano-silver had a cytotoxic effect at concentrations exceeding 15 ppm on PBMCs and 10 ppm on nasal epithelial cells. And less than 10 ppm of nano-silver has no cytotoxic effect, such as epithelial disarray or pigmentation [4].
Findings from the present study demonstrate that nano-silver instillation resulted in improvement of allergic symptoms and inhibition of OVA specific IgE antibody production in sensitized mice. In addition, Th2 cytokine (IL-4) levels in nasal lavage fluid are affected by nano-silver instillation. IL-10 is an anti-inflammatory cytokine produced by monocytes and to a lesser extent by lymphocytes. Although IL-10 is not directly associated with allergic inflammation, IL-10 suppression may lead to up-regulated expression of Th1 cytokines, like INF-γ, IL-3, granulocyte macrophage-colony stimulating factor (GM-CSF), and tumor necrosis factor (TNF). Th1 cells are defined by their ability to secrete the inflammatory cytokines IL-2, INF-γ and are involved in cellular immunity, some autoimmune diseases, and in chronic inflammatory disorders. Th2 biased cells preferentially produce IL-4, IL-5, and IL-13 and participate in humoral response and antibody production. In the present study, nano-silver was found to strongly affect Th2 oriented immune responses. Silver nanoparticles attenuate airway inflammation and hyperresponsiveness through modulation of reactive oxygen species generation, subsequently resulting in attenuated expression of inflammatory cytokines and NF-κB activation [16]. Anti-inflammatory properties of nanocrystalline silver with significantly suppressed expression of IL-1β, IL-12, metalloproteinase-9, and TNF-α have been demonstrated [17]. The mechanism of action of nano-silver particles was not evaluated in this study and it is difficut to come to a conclusion, nano-silver may inhibit NF-κB mediated cell activation. However, our results suggest that nano-silver inhibits nasal allergic inflammation through inhibition of cytokine production from inflammatory cells.
Allergic rhinitis exhibits sneezing, itching and watery rhinorrhea with tissue eosinophilia. Shin et al. [18] reported on increased thickness of lamina propria and epithelium in an allergic rhinitis mouse model. Although nano-silver had an anti-inflammatory effect, epithelial thickness was not significantly changed. In this experiment, nano-silver attenuated inflammatory cell infiltration and PAS-positive cells, which might result in improvement of nasal symptoms, such as sneezing, rubbing and nasal secretion in a mouse model. To decrease the thickness of nasal mucosa, long term inoculation of nano-silver might be needed.
The pharmacokinetics of the anti-inflammatory effect of nano-silver has not been studied and is not fully understood. Less than 10 ppm of Medisil, which was used in this experiment, has anti-allergic characteristics. By intranasal inoculation of nano-silver, Th2 inflammatory cytokine, which is one of the main allergic immune factors, was significantly decreased. And at the same time, inflammatory change of nasal mucosa also showed significant improvement. Although we need further study for clinical use, we can suggest that nano-silver may be a useful therapeutic strategy through their properties as anti-inflammatory agents.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0064844).
Notes
No potential conflict of interest relevant to this article was reported.