Galectin-1, -3, -7 Expressions in Congenital and Acquired Pediatric Cholesteatomas Compared to External Auditory Canal Skin
Article information
Abstract
Objectives
There is a classical distinction based on clinical criteria between acquired and congenital cholesteatomas. To determine if these two types of lesions show different immunohistochemical features, we have studied the expression patterns of three distinctive galectins (animal lectins implied especially in cellular proliferation and apoptosis) in both types of cholesteatomas and compared it to their expression patterns in external auditory canal skin.
Methods
Our study is based on nine acquired and eight congenital cholesteatomas, obtained from children during ear surgery. Six specimens of normal adult auditory meatal skin served as control. Specimens were analyzed by immunohistochemistry using monoclonal antibodies with galectin-1 and galectin-3, and a polyclonal antibody with galectin-7.
Results
We did not observe any differences in the galectin distribution pattern between congenital and acquired pediatric cholesteatomas. Compared to the control group, cholesteatomas present some particular features. There was no expression of galectin-1 and a lower expression of galectin-3 in the epithelium. Furthermore, we observed a preferentially nuclear distribution of galectin-7 in cholesteatomas, whereas it is essentially cytoplasmic in the control group.
Conclusion
The data reported in this study suggest, on the basis of a lesser marked galectin-3 in cholesteatomas epithelium compared with an external auditory canal skin, that an immature keratinocytes population is at the origin of these lesions and that galectin-3 and galectin-7 play a part in the capacity as apoptosis modulators. Our study does not establish a difference in the galectin expressions of congenital and acquired cholesteatomas, but it constitutes however an additional argument in favor of the "undifferentiated" origin of keratinocytes in cholesteatomas.
INTRODUCTION
Cholesteatoma is defined by a keratinizing squamous epithelium in the middle ear cavities. Although cholesteatoma is a benign disease, it can invade neighboring tissues and often recur even if surgical resection is considered to be complete. Cholesteatoma is traditionally classified as either acquired, essentially due to a chronic otitis process, or congenital. Congenital cholesteatoma are classically presented as a white mass behind an intact tympanic membrane, typically in the anterosuperior quadrant (1). Although acquired and congenital cholesteatoma are histologically identical, they do not share the same etiopathogenesis.
Congenital cholesteatoma is thought to occur due to secondary failure of normal involution of the epidermoid formation. This collection of stratified squamous epidermoid cells appears during fetal development (1). Many other mechanisms have also been proposed, such as metaplasic origins or a migration of epithelial cells from the external auditory canal (2, 3).
In the same way, the origin of acquired cholesteatoma remains under discussion. Among the various advanced theories, the most probable one considers the epithelial migration as the origin of the pathology. This migration can either start from the margins of a tympanic perforation, or from the retraction of the tympanic membrane (4).
The particularly aggressive behavior of cholesteatomas can be explained - at least partially - by disorders in growth regulation and cellular death of keratinocytes. The apoptosis of excessive keratinocytes is related to modifications of expression of various proteins, in particular the phosphoprotein p53 (5). Recurrent cholesteatomas can be distinguished from nonrecurrent on the basis of the quantity and the distribution from apoptotic cells. Among proteins implied in the regulation of this population of keratinocytes, galectine-3 seems to hold a particular role (6).
Galectins are members of an animal lectin family defined by shared consensus amino acid sequences and an affinity for ß-galactose-containing oligosaccharides. To date, 15 different galectins have been identified. They are implied in varied biological phenomena such as embryonic development, immune response, cellular proliferation or apoptosis. They also play a similar role to that of adhesion molecules on intercellular interactions and extracellular matrix-cell interaction (7-12).
Although, the expression of galectins has been observed in many normal and pathological tissues studies (including cholesteatomas for galectin-1, -3, and -8) (6), no previous study has been interested in the expression of these proteins in congenital and acquired cholesteatoma compared to external auditory canal skin.
Based on the observation that histologically similar tumors can present different immunohistochemical patterns (13), it seemed to us interesting to study the immunohistochemical behavior of congenital and acquired cholesteatomas on the basis of their expressions of galetin-1, -3, and -7 and to compare it with the expression patterns of these galectins in external auditory canal skin.
MATERIALS AND METHODS
Histopathologic and clinical data
Eight congenital cholesteatomas (six males, two females, average age 7.8 years) and 9 acquired pediatric cholesteatomas (eight males, one female, average age 9.6 years) were obtained immediately after middle ear surgery. In all cases, it was a first surgical cure of cholesteatoma. Six congenital cholesteatomas were obtained from the ENT Department of the "Reine Fabiola Children's Hospital" (Brussels, Belgium). The others were obtained from the ENT Department of the "Erasmus University Hospital" (Brussels, Belgium) and all were subjected to the standard diagnosis routine in the Department of Pathology of this same hospital. All the congenital cholesteatomas exhibited a classical otomicroscopic aspect for this disease, i.e., a white mass behind an intact tympanic membrane. Specimens of normal adult auditory meatal skin (n=6) obtained during autopsy served as controls. All autopsies were performed within a 24 hours postmortem delay to ensure a good preservation and staining of tissue antigens (14).
The specimens were immediately fixed in 4% formaldehyde and embedded in paraffin. Sections were cut at a thickness of 5 µm and processed for H&E staining using routine protocols. This study was approved by the ethical committee of the Erasmus University Hospital (Ref P2010/069).
Immunohistochemistry
The 5-µm-thick sections were subjected to standard immunohistochemistry, as previously detailed (15-17). The immunohistochemical expression was visualized by means of streptavidin-biotin-peroxidase complex kit reagents (BioGenex, San Ramon, CA, USA) with diaminobenzidine/H2O2 as the chromogenic substrate. Finally, the sections were counterstained with haematoxylin. Galectin-1 and galectin-3 expression was evidenced by means of two specific monoclonal antibodies, respectively (Novocastra, Newcastle, UK; dilution 1:100). Galetin-7 was immunolocalized in tissues with a polyclonal rabbit anti-human galectin-7 antibody, as detailed elsewhere (15). Negative controls had the primary antibodies replaced by non-immune serum (Dako, Glostrup, Denmark).
Immunohistochemistry: evaluation
We performed a semi-quantitative (for galectin-1 and galectin-7) and quantitative (for galectin-3) evaluation. For each slide, the entire tissue was analyzed. Two independent observers conducted on blind-labeled sections the assessment of the immunohistochemical staining of galectin-1 and -7.
The assessment of the immunohistochemical staining was considered as follows: 1) For galectin-1, according to the presence or the absence of staining within the stroma and/or the epithelium; 2) For galectin-3, a quantitative analysis is accomplished by computer-aided microscopy (×200 magnification; Histolab, Alphelys, France). The two studied variables were the labeling index (LI) and the index of transmitted intensity. The LI refers to the percentage of tissue area specifically stained by a given histochemical marker. The Index of transmitted intensity denotes staining intensity; 3) For galectin-7, the score was established after counting the number of labeled nuclei compared to all nuclei. The staining was considered nuclear if more than 10% of nuclei were labeled in the epithelium.
Statistical analysis
Due to the restricted number of cases and the obviously similar immunohistochemical staining in both of the cholesteatomas groups, we decided to compare directly the cholesteatomas (acquired and congenital) groups with the control group. Concerning the statistical analysis of the galectin-3 data, the differences between independent groups of quantitative data were analyzed by the Mann-Whitney non-parametric test. Concerning galectin-1 and -7 (two binary variables), Fisher's exact test were used. All the statistical analyses were carried out using Statistica (Statsoft, Tulsa, OK, USA).
RESULTS
We did not observe any differences in the galectin distribution pattern between congenital and acquired pediatric cholesteatomas (Table 1).

Galectin-1, -3, and -7 distribution patterns in external auditory canal (EAC) skin, acquired and congenital cholesteatoma
For galectin-1, a significant difference (P=0.00002, Fisher's exact test) is observed between the cholesteatoma and the external auditory canal skin group. In fact, cholesteatoma's epithelium does not express galectin-1, unlike the external ear skin specimens. Each specimen from both groups presented a stromal staining for galectin-1 (Fig. 1).
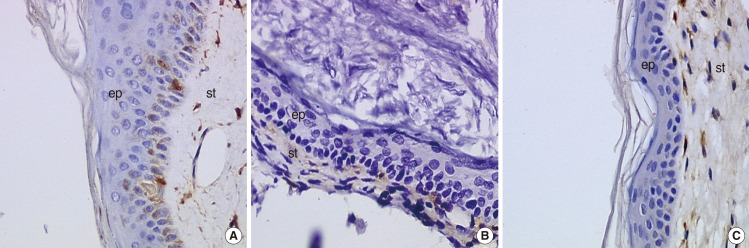
Morphological aspect of the immunohistochemical staining for galectin-1 (×400). (A) External auditory canal, (B) acquired cholesteatoma, (C) congenital cholesteatoma. st, ststroma; ep, epithelium.
For galectin-3, the LI (P=0.0033, Mann-Whitney non-parametric test) as well as the index of transmitted intensity (P=0.0026) are significantly different between both groups (Fig. 2). Indeed, external auditory canal skin presented an intense and wider galectin-3 staining compared with acquired and congenital cholesteatomas. The staining in the external auditory canal skin group was particularly intense at intercellular junctions (Fig. 3).
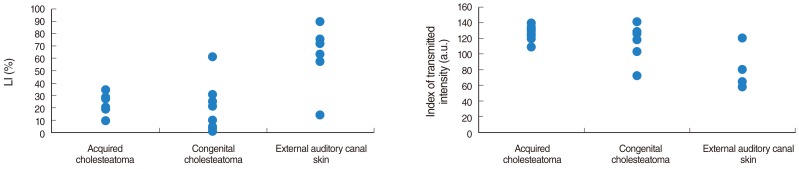
Labeling index (LI) and index of transmitted intensity for galectin-3 in the three histological groups.
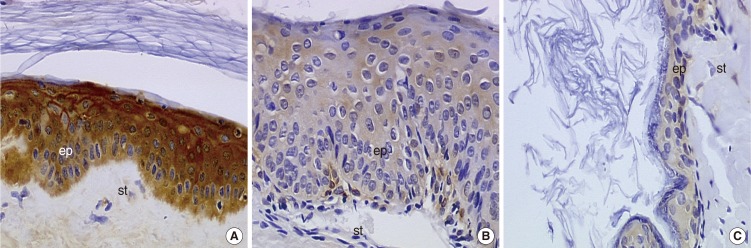
Morphological aspect of the immunohistochemical staining for galectin-3 (×400). (A) External auditory canal, (B) acquired cholesteatoma, (C) congenital cholesteatoma. st, ststroma; ep, epithelium.
For galectin-7, the cholesteatoma group (acquired and congenital) presented a predominant nuclear staining, while the control group presented a cytoplasmic predominant staining (Fig. 4). Moreover, the distribution of cells whose nucleus was positive was distributed heterogeneously, with some areas rich in labeled nucleus. This however, was statistically relevant (P=0.00009, Fisher's exact test).
DISCUSSION
The main result of this study is that acquired and congenital cholesteatomas exhibit the same galectin distribution patterns. The comparative analysis of the immunohistochemical behavior of these two forms of cholesteatomas is an interesting method to obtain information on their possible origins. Although a distinction between the two types of cholesteatomas could be recently established on the basis of difference in length of telomeres (18), there is currently no other evidence that these two groups of cholesteatomas show a difference in biological properties. For example, the cytokeratins expression does not differ according to whether the cholesteatoma is congenital or acquired (19). Our study reaches the same conclusion regarding the expressions of galetin-1, -3, and -7.
Galectins and cholesteatomas
If the presence of galetin-1 and -3 is already documented in skin and cholesteatomas (6, 20), no study has yet looked at galectins expression analysis in cholesteatomas in comparison with skin from the external auditory canal. Moreover, galectin-7 has never been studied in cholesteatoma. Galectin-1 is expressed in many cellular types and plays a role in varied biological phenomena, such as proliferation, apoptosis and cellular adhesion (7-9, 20). Contrary to what had been noted by Sheikholeslam-Zadeh et al. (6), which had shown a marked presence of galectin-1 in cholesteatoma epithelium, we have observed an absence of marking of galectin-1 within cholesteatoma epithelium. But their stromas, as well as the epithelium and the stromas of external auditory canal skin, expressed in galectin-1. These contradictory results are probably explained by a difference between the antibodies used: we employed a monoclonal anti-galectin-1 antibody, whereas a polyclonal antibody was used in their experiments. The monoclonal antibodies, resulting from a lymphocytary clone and thus directed against only one epitope, are more specific - but also often less sensitive than polyclonal ones (21). This difference between the two studies would thus result either from a lack of specificity of the polyclonal antibodies, or of a lack of sensitivity of the monoclonal ones.
Although, we know that the expression of galectin-1 in the epidermal layer evolves during embryogenesis and carcinogenesis (22), it is difficult to clarify the role of galectin-1 in cholesteatomas. Indeed, as in cholesteatoma, the studies seem contradictory concerning the presence of galectin-1 in epidermis (23, 24).
Our study shows that galectin-3 is less expressed, both on the level of the surface marked and on the level of the intensity of marking, in cholesteatoma epithelium compared with the external auditory canal skin. It was recently shown that the expression of galectin-3 increased during keratinocytes differentiation (20). This observation pleads in favor of an "undifferentiated origin" of keratinocytes in cholesteatomas. In the same way, it was suggested, on the basis of expression of the retinoic acids receptors in cholesteatomas that an immature keratinocyte population is at the origin of cholesteatoma lesions (25). Haake and Cooklis (26) noted an important apoptosis rate in fetal keratinocytes. Moreover, they have suggested that apoptosis was a borrowed way by keratinocytes when their differentiation were not complete. An abnormally high rate of apoptosis also precisely characterizes cholesteatomas (5). This information is interesting, as the galectine-3 is known for its anti-apoptotic effect (10), especially in keratinocytes (27). It is interesting to note also that in malignancies conditions, the levels of expression of galectin-3 decreased as the levels of differentiation decreased (28). Furthermore, the same galectin-3 pattern expression is found in psoriasis, another keratinocyte pathologic condition (24).
We have observed a preferentially nuclear distribution of galectin-7 in cholesteatomas. Galectin-7 is known to display pro-apoptotic effects: it has been shown to be one of the genes whose expression is induced in the early steps of p53-mediated apoptosis (29). As proposed by Kuwabara et al. (30), galectin-7 would present pro-apoptotic properties when localized intracellularly, and that these effects should be induced in the nucleus rather than in the cytoplasm. It is also interesting to note that the expression of galectin-7 in hypopharyngeal and laryngeal dysplasia is characterized by a shift from the cytoplasmic compartment (normal epithelium) to the nucleus (dysplasia) (22).
It remains difficult to allot a well-defined role to galectins in regulation phenomena that proceed in cholesteatomas, and this is due to two reasons. The first one is related to galectins own properties. Galectins are implied in a very large amount of biological phenomena - sometimes opposites. They do not depend only on the type of cell in which they take place, but also on their localization (nuclear, cytoplasmic or extracellular) (8, 10, 12), which makes there interpretation difficult. The second reason is due to the small size of our samples.
To conclude, the data reported in this study suggest that on the basis of a lesser marked galectin-3 in cholesteatomas epithelium compared with an external auditory canal skin that an immature keratinocytes population is at the origin of these lesions and that galectin-3 and galectin-7 play a part in the capacity as apoptosis modulators. If our study does not establish a difference in the galectin expressions of congenital and acquired cholesteatomas, it constitutes however an additional argument in favor of the "undifferentiated" origin of keratinocytes in cholesteatomas.
To establish more precisely the role of galectins and the factors influencing their expression in cholesteatomas, and to determine possible biological differences between congenital and acquired specimens, a broader study with a larger sample group would be necessary.
Notes
No potential conflict of interest relevant to this article was reported.
