Decreased Expression of TLR-9 and Cytokines in the Presence of Bacteria in Patients with Otitis Media with Effusion
Article information
Abstract
Objectives
Toll-like receptor (TLR)-9 recognizes unmethylated cytidine-phosphate-guanosine (CpG) motifs in bacteria. Therefore, the expression of TLR-9 may differ according to the results of bacterial culture, and thus a change in proinflammatory cytokine induction can also be expected. The authors aimed to assess the differences and relationships between the expression of TLR-9, cytokines, and nitric oxide synthase (NOS) in otitis media with effusion (OME) based on bacterial culture results.
Methods
Sixty-eight patients with OME were divided into culture-positive and culture-negative groups based on middle ear culture results. mRNA expression of TLR-9, NOS, and cytokines was measured and analyzed.
Results
Bacteria were detected in 38.2% of patients, and the distribution was as follows: coagulase negative Staphylococcus (10.3%), Staphylococcus aureus (8.8%), Streptococcus pneumonia (5.9%), and Bacillus spp. and Haemophilus influenza combined (2.9%). There were no significant differences in epidemiologic characteristics according to the culture results. Down-regulation of TLR-9 was observed in the culture-positive group (P=0.019). Cytokines including interleukin (IL)-12 (r=-0.582), tumor necrosis factor (TNF)-α (r=-0.569), interferon (IFN)-γ (r=-0.442), IL-6 (r=-0.395) and inducible NOS (r=-0.256) tended to decrease with the detection of bacteria.
Conclusion
The expression of TLR-9 significantly decreased in OME with confirmed bacterial pathogens. IL-12, TNF-α, IFN-β, IL-6 expression tended to decrease with the detection of bacteria. The presence of bacterial pathogens in OME may be related to abnormalities in the innate immune system.
INTRODUCTION
Otitis media with effusion (OME) is defined as the presence of fluid in the middle ear without signs or symptoms of acute infection. Sixty percent of children experience OME by two years of age, and approximately 90% of children have had OME before they enter school [1].
It is generally accepted that dysfunction or underdevelopment of the eustachain tube, prior upper respiratory viral infection and subsequent bacterial infection, allergies, parental smoking, and daycare classrooms with more than six students are associated with the development of OME [2-4]. Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis are commonly detected in the middle ear fluids of patients with OME; however, 40%-60% of OME cases have culture-negative results [5].
The relationship between bacterial culture results and immunity is controversial. An earlier study reported that in patients aged 10-18 months, there was a decreased likelihood of isolating bacteria from middle ear fluid if the patient had higher concentrations of IgA in the middle ear fluid than in the serum [6]. These results highlight the importance of local immunity in middle ear inflammation. However, other studies have emphasized the importance of systemic immunity, showing that bacterial cultures do not correlate with concentrations of immunoglobulins in the middle ear, but are related to concentrations of immunoglobulins in the serum [7].
Innate immunity is the first line of the human immune defense mechanism against bacterial invasion prior to initiation of adaptive immunity, and innate immunity is responsible for the initial host response against infection [8]. Molecular characteristics of pathogens are recognized by pattern-recognition receptors (PRRs), which induce the release of cytokines and chemokines and prompt the mobilization of interferon. Examples of PRRs include toll-like receptors (TLRs), cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors, and retinoic acid-inducible gene (RIG)-I.
One recent study reported that levels of TLR-9, NOD-1, and RIG I mRNA expression were significantly lower in an otitis-prone group and that the decreased expression of PRRs may be related to increased susceptibility to OME [9].
TLR-9 recognizes unmethylated cytidine-phosphate-guanosine (CpG) motifs in bacteria and is associated with activation of B cells [10]. TLR-9 is also a significant mediator of inflammation at other sites and synergizes with other TLRs involved in the pathogenesis of otitis media [11]. These characteristics explain why TLR-9 is important in evaluating innate immunity against bacterial infections of the middle ear.
Based on these facts, the authors propose that expression of TLR-9 in OME may differ according to bacterial culture results, thereby affecting proinflammatory cytokine release as well. To test this hypothesis, mRNA expression levels of TLR-9, cytokines, and nitric oxide synthase (NOS) were assessed using middle ear fluid collected from children with OME.
MATERIALS AND METHODS
Patients
From May 2009 to May 2011, 68 children who were diagnosed with OME and underwent tympanostomy tube insertion were enrolled in this study. During each patient's first visit, a detailed medical history was obtained, and physical examinations were performed including anterior rhinoscopy, otoscopy, impedance audiometry, pure tone audiometry or speech audiometry. OME was diagnosed by the presence of an amber colored tympanic membrane on otoscopic examination or by the presence of B or C type tympanograms as indicated by impedance audiometry.
Surgery was performed on patients who did not show improvement after three months, who experienced progressive retraction of the eardrum, or who demonstrated progressive hearing loss as suggested by an increase in pure tone threshold. Prior to surgery, the external auditory canal was washed with potadine solution, a radial incision was made in the anterior inferior quadrant of the tympanic membrane, and a tympanostomy tube was inserted. Middle ear effusion fluid (MEEF) was aspirated using a collector (Xomed Trace Products, Jacksonville, FL, USA) employing aseptic procedures and ensuring that bleeding was avoided. Fluid samples were transferred to Eppendorf tubes and stored at -80℃.
The purpose of the study was explained and written informed consent was obtained from a parent or guardian of each patient. The study was approved by the Ethics Committee of Kyung Hee University Hospital. Children who were suspected to have acute otitis media (AOM), who had head-and-neck anomalies, who had systemic diseases, or who had a congenital or an acquired immunodeficiency, were excluded.
Bacterial culture
Each effusion fluid sample was collected using a sterile cotton swab (Xomed Trace Products) and each swab was immersed in Stuart transport medium. These samples were used to inoculate blood agar and thioglycollate liquid media. All cultures were incubated for at least 24 hours at 35℃, and bacterial colonies were identified by Gram staining and biochemical analysis.
Real-time PCR
Total RNA was extracted from the effusion fluid using the RNA-Bee solution kit (Tel-Test, Friendswood, TX, USA) following the manufacturer's protocol. First-strand cDNA synthesis was performed by reverse transcription in a total volume of 20 mL reaction mixture containing 1 mg of RNA, 1x reaction buffer, 1 mM dNTPs, 5 µM random primers, 20 units of RNase inhibitor, and 20 units of AMV reverse transcriptase (Promega, Madison, WI, USA).
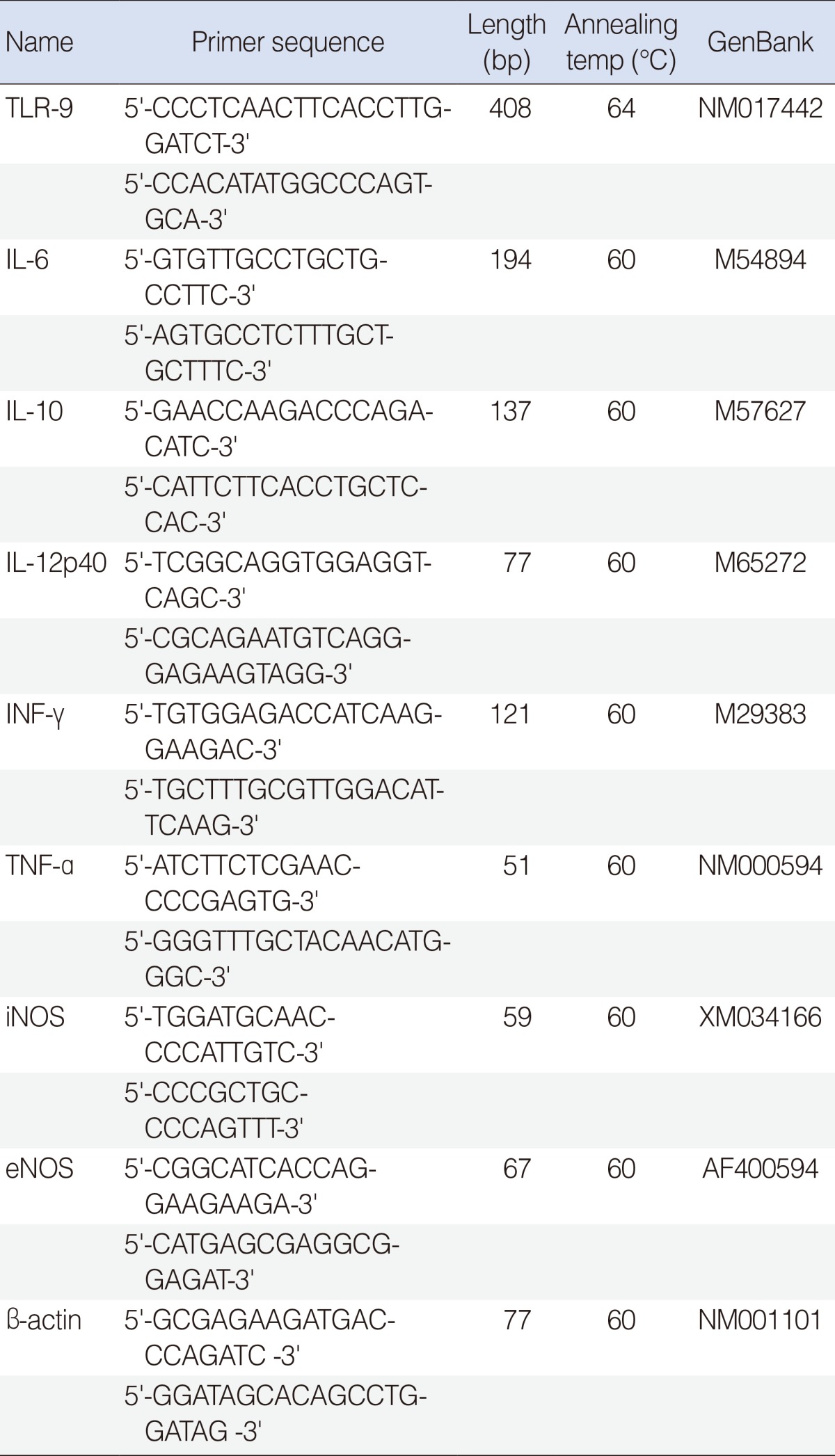
The reaction mixture was incubated at 42℃ for 1 hour and terminated by heating at 95℃ for 5 minutes. TLR-9, cytokines (interleukin [IL]-6, -10, -12, tumor necrosis factor [TNF]-α, interferon [IFN]-γ), and nitric oxide primers are shown in Table 1. Real-time polymerase chain reaction (PCR) was performed using a Chromo4 Detector real-time system (Bio-Rad, Hercules, CA, USA) with the SsoFast EvaGreen supermix (Bio-Rad). PCR was performed with 2 µL of cDNA in a 20 µL reaction mixture of 10 mL SsoFast EvaGreen supermix, 2 mL primers and 6 mL PCR grade water. The reaction parameters were as follows: denaturation at 95℃ for 30 seconds followed by 45 cycles at 95℃ for 5 seconds, and 55℃ to 64℃ for 12 seconds. The crossing point of TLR-9, cytokines (IL-6, -10, -12, TNF-α, IFN-γ), and nitric oxide with β-actin was applied to the following formula: 2 - (target gene - β-actin) and relative amounts were quantitated.
Statistical analysis
Patients were classified into two groups according to bacterial culture results, and levels of TLR-9, cytokine, and nitric oxide expression were assessed. Statistical comparisons were made by the Kruskal-Wallis test and the Mann-Whitney U-test to identify differences in numeric data, and Fisher exact test was used for nominal data. Correlations between PRRs, cytokines and the presence of bacteria were analyzed by polyserial analysis. P-values less than 0.05 were considered statistically significant.
RESULTS
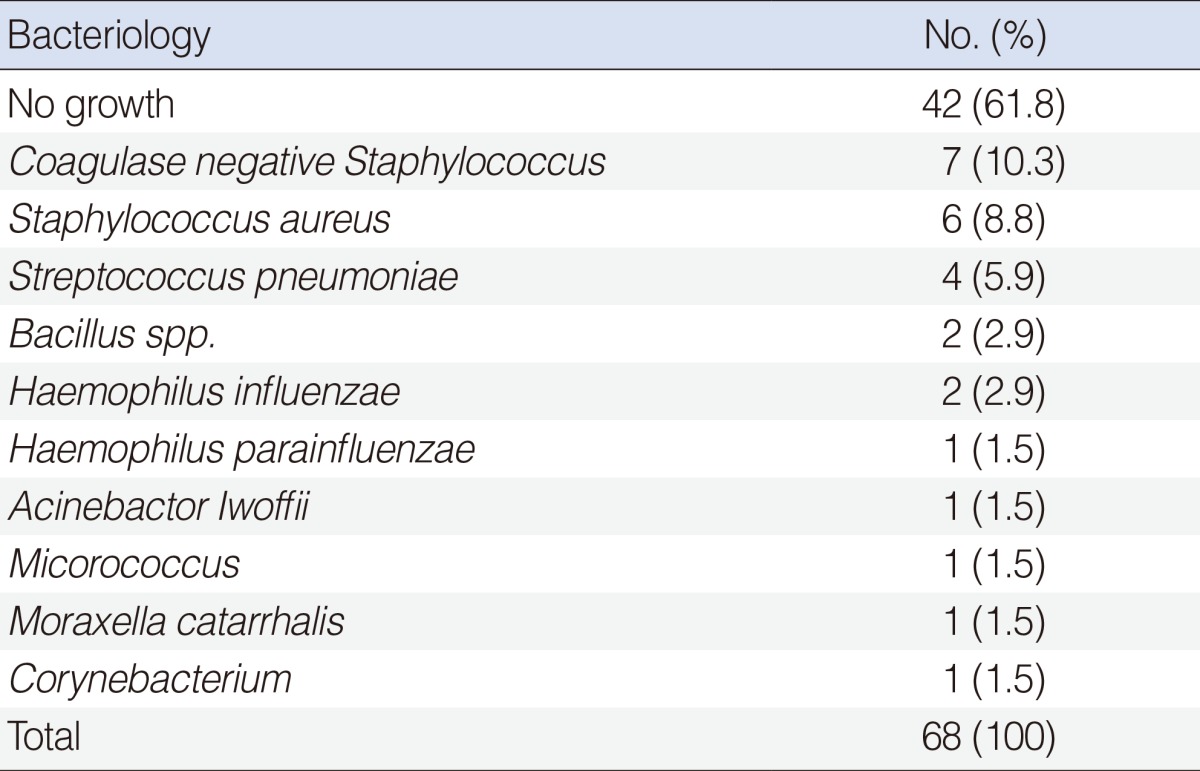
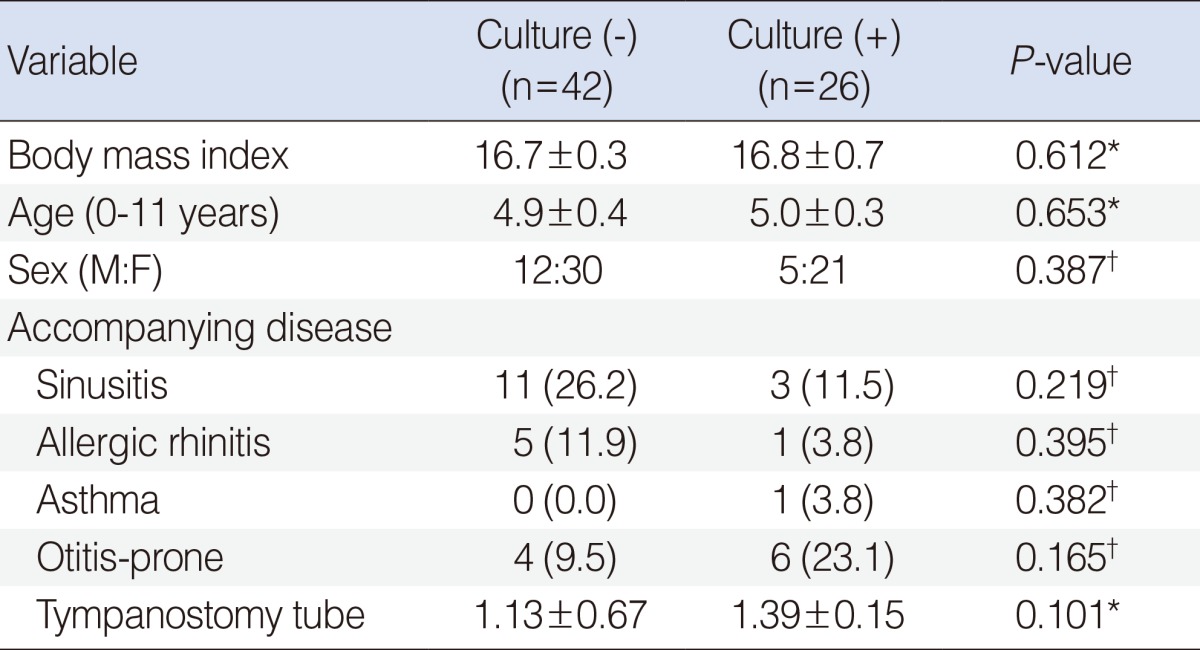
Sixty-eight patients were included in the study, with 51 males and 17 females. The mean age was 4.9±2.1 years (range, 0 to 11 years). Bacteria were detected in 38.2% of patients (n=26). Coagulase negative Staphylococcus (CNS) was detected in 10.3% of the patients, S. aureus in 8.8%, S. pneumoniae in 5.9%, and Bacillus spp. and H. influenzae in 2.9% (Table 2). When patients were classified into two groups according to culture results, there was no significant difference in age, sex, body mass index, frequency of tympanostomy tube insertion, or accompanying diseases between groups (P>0.05) (Table 3).
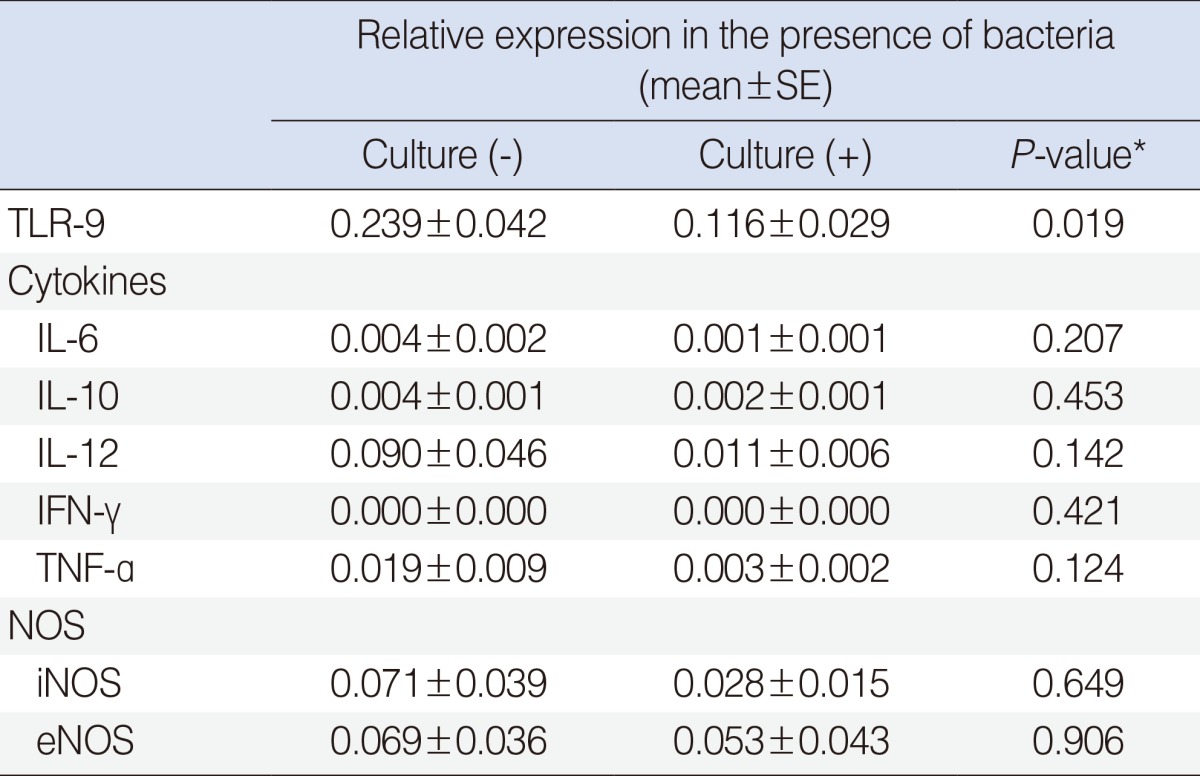
Polyserial correlation analysis revealed that increasing tendencies of bacteria were significantly correlated with decreased mRNA expression of TLR-9, cytokines and NOS (P<0.05) (Table 4). Of these, TLR-9 (r=-0.399), IL-12 (r=-0.582), TNF-a (r=-0.569), IFN-γ (r=-0.442), and IL-6 (r=-0.395) showed significant correlation more than low negative (Table 4). When mRNA expression levels were compared directly according to culture results, the expression of TLR-9 mRNA was significantly lower in the culture-positive group than in the culture-negative group (P=0.019) (Table 5). There were no significant differences in the expression of cytokines or NOS between the culture-positive and culture-negative groups (P>0.05).
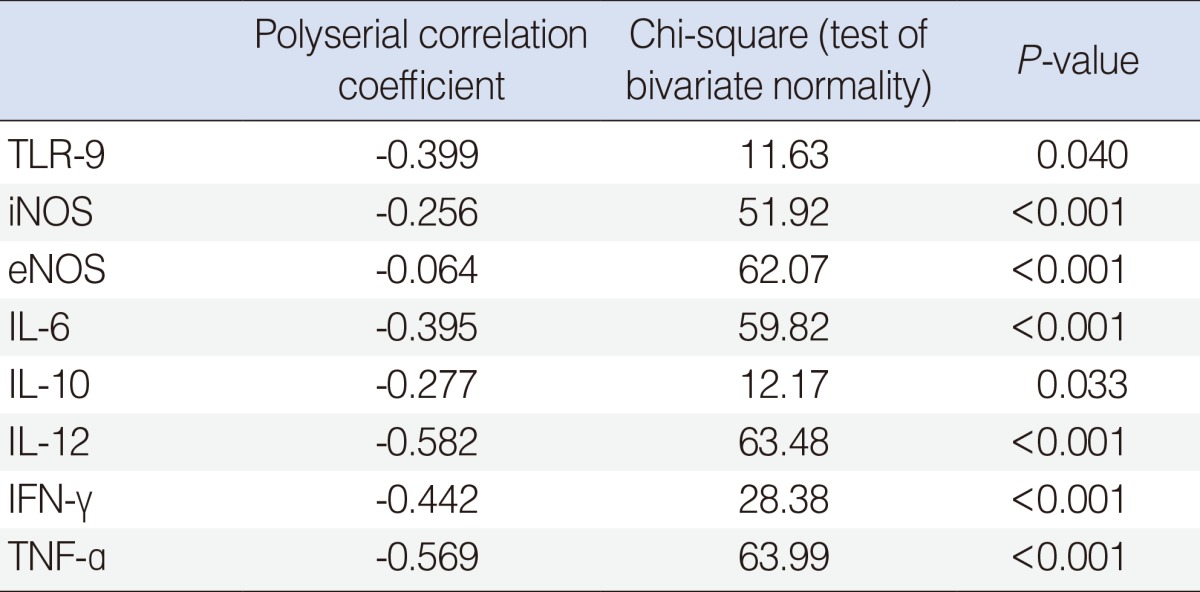
Polyserial correlation analysis between the expression of PRRs, cytokines and the presence of bacteria
DISCUSSION
Unexpectedly, CNS was the most common pathogen found (Table 2). Typically, H. influenzae, S. pneumoniae, and Moraxella catarrhalis are the three most common pathogens detected in the OME patients [5]. In the past, CNS has been regarded as a mere nonpathogenic commensal organism of the external auditory canal. However, recent studies have shown that this bacterium can infect biomaterials, forming biofilms. The bacterium also causes endocarditis, wound infections, and otitis media [12,13]. Interestingly, recent studies have shown that CNS is more prevalent in MEEF than are the three traditional pathogens. This may be because of variation in bacterial prevalence over time, antibiotic use, and/or vaccination against H. influenzae and S. pneumoniae [12,14].
mRNA expression of TLR-9 decreased significantly in the culture-positive group compared to the culture-negative group. TLRs, a type of PRR, detect specific molecules related to microbial pathogens and act as the host's first line of defense against infection [4]. TLRs can be classified into two subgroups according to cellular localization and their respective pathogen-associated molecular patterns (PAMP) ligands. TLR-1, -2, -4, -5, -6 are expressed on the cell surface and primarily recognize microbial membrane components such as lipids, lipoproteins, and proteins. On the other hand, TLR-3, -7, -8, -9 are expressed in the intracellular vesicle, including the endoplasmic reticulum, endosomes, lysosomes, and endolysosomes, and primarily recognize microbial nucleic acid [15,16].
In this study, TLR-9 initiated the immune response after recognizing the unmethylated CpG motifs in bacteria. Self-limiting AOM deteriorates into persistent otitis media or results in abnormal recovery when there are TLR deficiencies or genetic defects related to TLR pathways [4,17]. A recent study indicated that bacterial pathogens were detected in 64% of patients diagnosed with AOM [18]. Because OME is likely to occur after resolution of AOM, the fact that bacteria were found in samples even after three months can be explained by the fact that abnormalities or deficiencies in innate immunity might cause problems with acquired immunity or may complicate cytokine induction in the middle ear, resulting in an increased likelihood of bacterial presence. Abnormalities in innate immunity can be caused by single nucleotide pleomorphisms (SNPs), down-regulation of the innate immune response by infection [19,20], and accompanying autoimmune diseases. In addition to responding to PAMPs, endogenous host molecules such as the degradation products of extracellular matrix components, heat-shock proteins, and high-mobility group box 1 proteins produced by cell death or injury can stimulate cell surface TLRs and ultimately cause systemic autoimmune disease [16].
In this study, the likelihood of undiagnosed autoimmune disease may not have been high because not all of the patients had an unusual medical history, other than the OME. However, physicians should still keep in mind the possibility of autoimmune disease accompanying OME. In summary, decreased expression of TLR-9 in the middle ear may be related to increased susceptibility to bacterial OME.
Levels of IL-12, TNF-a, IFN-r, and IL-6 expression were not significantly different based on the type of bacteria present. However, all of these expression levels tended to decrease with increasing identifications of bacteria. IL-12 is a potent inducer of IFN-γ and plays an important role in the development of OME regardless of allergic status [21]. TNF-α is known to be a strong inducer of IL-10, IL-6 production. IL-6 is the secondary cytokine secreted by IL-1β and TNF-α. IL-6 stimulates antibody production similar to IL-1β and is associated with bone resorption. Additionally, IL-6 down-regulates the production of the pro-inflammatory cytokine IL-10.
Most TLRs, with the exception of TLR-3, use the myeloid differentiation factor-88 (MyD88) pathway via activation of nuclear factor-Kb, and produce proinflammatory cytokines such as TNF-α, IL-1, and IL-6. The MyD88-independent Toll/IL-1 receptor (TIR)-domain-containing adaptor inducing IFN-β (TRIF) pathway induces delayed cytokine gene expression via the production of type I. TNF in particular is known to regulate TLRs via feedback mechanisms and thus modulates the complex networks of downstream cytokines [4,22]. The tendency of both TLR-9 and cytokine expression to decrease with increasing detections of bacteria simultaneously suggests the failure of innate immunity.
Inducible NOS (iNOS) expression also tended to decrease with positive culture results (r=-0.256), although expression levels were not significantly different between the culture-positive and culture-negative groups (P>0.05). Nitric oxide (NO) is secreted by the neurons, endothelial cells, platelets and neutrophils in response to homeostatic and pathologic stimuli. NOS is composed of three isoenzymes: endothelia NOS (eNOS), neuronal NOS (nNOS), and iNOS. Of these, eNOS and nNOS are constitutively expressed. However, iNOS is normally absent in macrophages, is upregulated by stimulation, and is involved in killing pathogens or vasodilation [23,24]. Because the activation of iNOS is dependent on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and signal transducer and activator of transcription (STAT)-1α pathways [25], the fact that iNOS expression decreases as bacteria isolations increase may be related to the down-regulation of cytokine release.
One limitation of this study is that the possibility of genetic variations, including SNPs, was not addressed. Recent studies have reported an association between TLR-9 SNPs and various diseases including systemic lupus erythematous, bacterial meningitis, atherosclerosis, and asthma. However, reports concerning OME are still rare, so additional research regarding the relationship between SNPs, OME susceptibility and culture results may enhance the understanding of OME pathogenesis.
In addition, children with early stage OME were not included in this study. The exudates used in this study were collected during surgery two to three months after the initial onset of otitis media. This made it impossible to determine the levels of TLR-9, cytokine, NOS, and transcription factor, and immunoglobulin mRNA expression at the onset of otitis media. Furthermore, for ethical reasons, it was not possible to collect middle ear mucosa from patients in this study and therefore experiments were performed on exudates. These exudates contained only a few partially exfoliated mucosal epithelial cells, inflammatory cells, and immune cells and thus do not fully reflect the immune response in the middle ear mucosa during infection.
Finally, the low detection rates afforded by conventional culture also limit the findings of our present study. Only 38.2% of cultures were positive. The activities of host defense mechanisms, antibiotic treatment prior to collection, the presence of bacterial strains that cannot be identified by conventional culture methods, and growth of bacteria as biofilms, may all contribute to the low detection rate. In efforts to overcome the shortcomings of conventional culture, use of the reverse transcriptase-PCR, multiplex PCR, and multi-locus sequence typing, is becoming more common. These techniques can identify pathogens in culture-negative fluid [26]. Further application of these methods may help elucidate the etiology of otitis media with effusion.
In conclusion, the expression of TLR-9 significantly decreased in OME with the presence of bacterial pathogens. IL-12, TNF-α, IFN-β, and IL-6 expression tended to decrease with the detection of bacteria. The presence of bacterial pathogens in OME may be related to innate immunity abnormalities.
ACKNOWLEDGMENTS
This research was supported by the Kyung Hee University Research Fund in 2011 (KHU-2011-1098).
Notes
No potential conflict of interest relevant to this article was reported.