Conversion from Selective to Comprehensive Neck Dissection: Is It Necessary for Occult Nodal Metastasis? 5-Year Observational Study
Article information
Abstract
Objectives
To compare the therapeutic results between selective neck dissection (SND) and conversion modified radical neck dissection (MRND) for the occult nodal metastasis cases in head and neck squamous cell carcinoma.
Methods
Forty-four cases with occult nodal metastasis were enrolled in this observational cohort study. For twenty-nine cases, SNDs were done and for fifteen cases, as metastatic nodes were found in the operative field, conversion from selective to MRNDs type II were done. Baseline data on primary site, T and N stage, extent of SND, extracapsular spread of occult metastatic node and type of postoperative adjuvant therapy were obtained. We compared locoregional control rate, overall survival rate and disease specific survival rate between two groups.
Results
Among the 29 patients who underwent SND, only one patient had a nodal recurrence which occurred in the contralateral undissected neck. On the other hand, among the 15 patients who underwent conversion MRND, two patients had nodal recurrences which occurred in previously undissected neck. According to the Kaplan Meier survival curve, there was no statistically significant difference for locoregional control rate, overall survival rate and disease specific survival rate between two groups (P=0.2719, P=0.7596, and P=0.2405, respectively).
Conclusion
SND is enough to treat occult nodal metastasis in head and neck squamous cell carcinoma and it is not necessary to convert from SND to comprehensive neck dissection.
INTRODUCTION
Selective neck dissection (SND) has been the standard surgical treatment for the patients with a clinically determined node negative neck, but whose primary head and neck cancer has a relatively high chance of occult nodal metastases [1]. In contrast, comprehensive neck dissection either radical or modified radical neck dissection (MRND), is mainly used for the clinically determined node positive neck [2].
Yet, in recent times, because of the morbidity of comprehensive neck dissection, with a better understanding of the patterns of cervical nodal metastasis and the development of an adjuvant therapy, SND has been more often performed for treating cervical lymph node disease in selected patients [3]. But the application of SND for therapeutic purposes remains unclear and controversial [4-6]. For this reason, conversion from SND to comprehensive neck dissection is sometimes performed, and especially when finding a positive neck node in the operative field that couldn't be found in the preoperative radiologic evaluation.
In this study, we tried to compare the locoregional control rate, overall survival rate and disease specific survival rate between SND and conversion from SND to MRND for the preoperatively node negative but intraoperatively or postoperatively node positive patients.
MATERIALS AND METHODS
Study design and the analyzed factors
The patients with head and neck squamous cell carcinoma who underwent surgical treatment as their 1st treatment at Ilsong Memorial Institute of Head and Neck Cancer, Hallym University Medical Center, Seoul, Korea from 2000 to 2006 were enrolled in this study. The study protocol was approved by Hallym University Medical Center Institutional Review Board, and all study participants signed written informed consent. The eligibility criteria included the patients who were preoperatively node negative by evaluation with computed tomography (CT), positron emission tomography (PET)/CT and ultrasound but final pathologic results after neck dissections were node positive. Among 44 cases, preoperatively designed SNDs were done in 29 cases as there was no suspicious node in the operative field. In contrast, as for 15 cases, conversion from SND to MRNDs type II were done as suspicious metastatic nodes were found in the operative field and the result of frozen biopsy was positive. Patients with a second primary tumor or a distant metastasis were excluded in this study.
Baseline data on primary site, T and N stage, extent of SND, extra-capsular spread of metastatic node and type of postoperative adjuvant therapy were obtained. We used the Kaplan Meier method to compare the locoregional control rate, overall survival rate and disease specific survival rate between two groups.
The primary site and the T&N stage
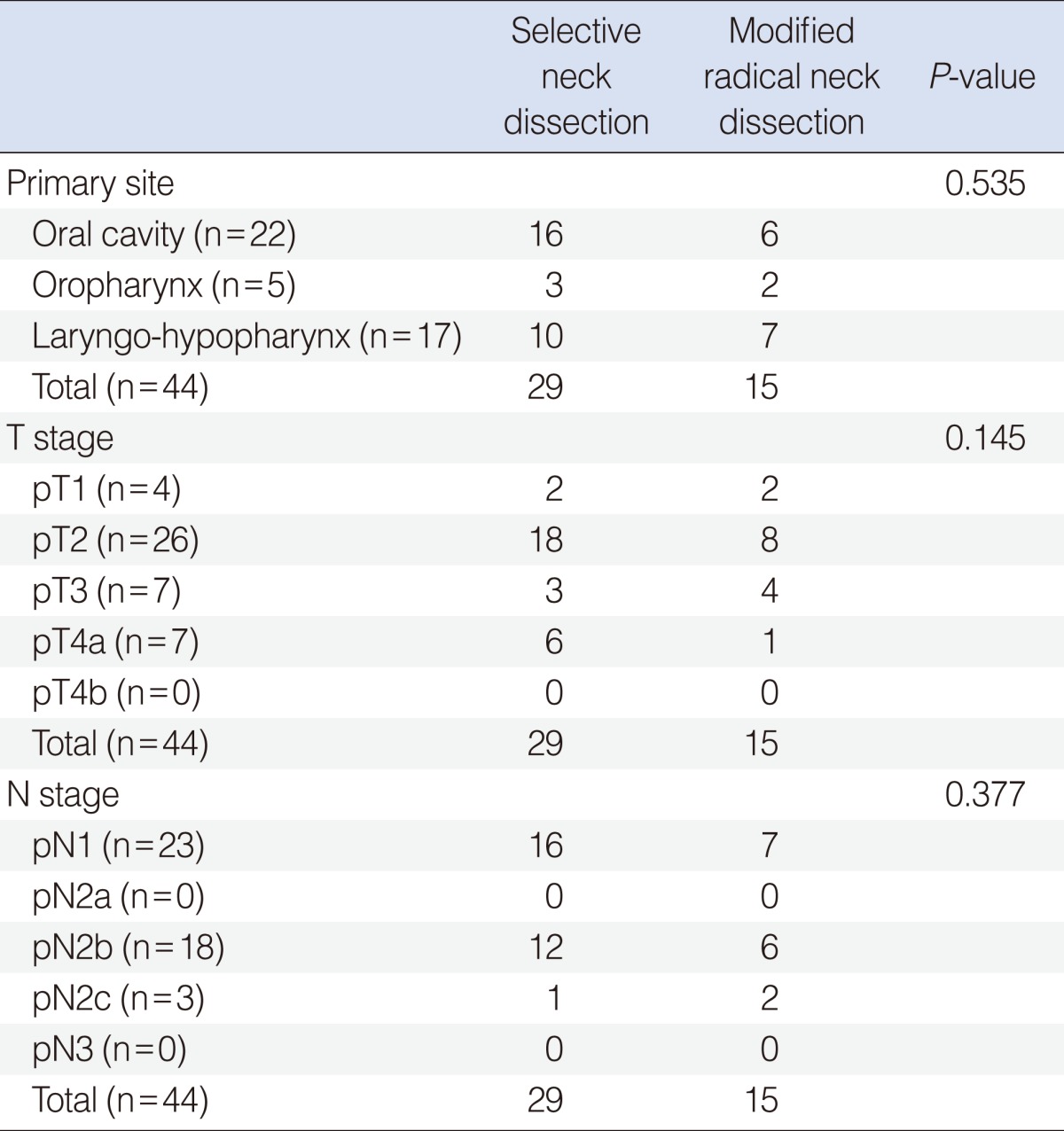
The oral cavity was the most common primary site in both the SND and MRND groups (n=16 and n=6, respectively) (Table 1). Table 1 also shows the pathological T and N stage distribution. There was no statistically significant difference for the primary site or the T and N distribution between the SND and MRND groups.
The profile of the neck dissections
In the SND group, SND (I-III) and SND (II-IV) were the most common types of neck dissection (n=12 and n=11, respectively) (Table 2). As for the MRND groups, all patients underwent conversion MRND type II (Table 2).
Selection of suspicious lymph node for frozen biopsy
After exposing whole neck contents, lymphatic tissue in each neck level was gently palpated. Any suspicious lymph nodes such as enlarged (>15 mm) or hard or conglomerated lymph nodes were excised and sent to pathologic department for frozen biopsy. The technique that was used in the frozen section examination was performed with a standard preparation with hematoxylin and eosin staining as used at our facility.
In this study, we included only the cases which frozen biopsy results were correlated with the results of final pathologic report. In other words, false positive cases of frozen biopsy results were excluded.
Extracapsular spread of metastatic node
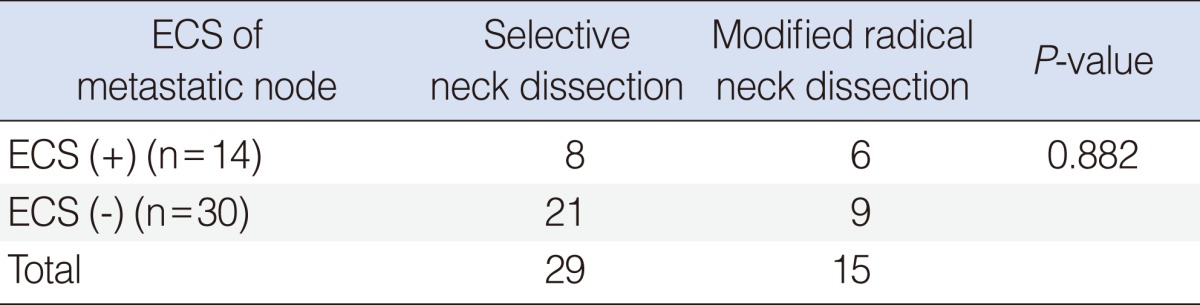
Etracapsular spread of metastatic node was found for 8 cases in the SND group and for 6 cases in the MRND group (Table 3). The number of metastatic node with extracapsular spread didn't show any statistically significant difference between two groups.
Postoperative adjuvant therapy
Postoperative radiation therapy (RT) or concurrent chemo-radiation therapy (CCRT) was done for 20 cases in the SND group and for 11 cases in the MRND group (Table 4). The eligibility criteria for postoperative CCRT included advanced T3 or T4 stage with close resection margin (<5 mm), multiple nodal metastasis or extracapsular spread of metastatic node. Postoperative RT was applied for early stage cancer with multiple nodal metastasis or extracapsular spread of metastatic node. The number of the patients who received postoperative adjuvant therapy didn't show any statistically significant difference between two groups.
Follow-up and the statistical method
The follow-up period ranged from 6 to 118 months (mean follow-up, 55 months). We used the Kaplan Meier method to compare the locoregional control rate, overall survival rate and disease specific survival rate between the SND group and the MRND group.
RESULTS
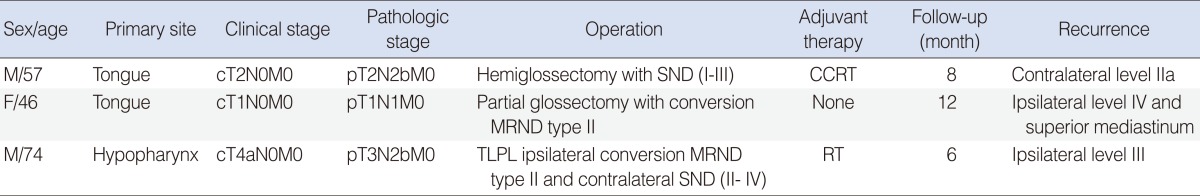
Among the 29 patients who underwent the SND, only one patient had a nodal recurrence which occurred in previously dissected neck. This patient was diagnosed as tongue cancer with stage cT2N0M0 and underwent hemiglossectomy and ipsilateral SND (I-III) but pathologic stage was pT2N2bM0. Eight months later, nodal recurrence was found in the contralateral level IIa (Table 5). The patient underwent MRND type II for contralateral side but after 5 months, died of locoregional recurrence with uncontrollable bleeding.
On the other hand, among the 15 patients who underwent MRND, two patients had nodal recurrences, which occurred in previously undissected neck. One patient underwent partial glossectomy with ipsilateral conversion MRND type II for tongue cancer pT1N1M0 but 12 months later, multiple nodal recurrences were found in the ipsilateral level IV and superior mediastinum. The patient underwent revision neck dissection and superior mediastinal dissection through transclavicular approach but after 8 months, died of locoregional recurrence.
The other patient underwent total laryngectomy, partial phryngectomy, ipsilateral conversion MRND type II and contralateral SND (II-IV) for hypopharyngeal cancer pT3N2bM0 but 6 months later, nodal recurrence was found in the ipsilateral level III. The patient underwent revision neck dissection but after 7 months, died of locoregional recurrence (Table 5).
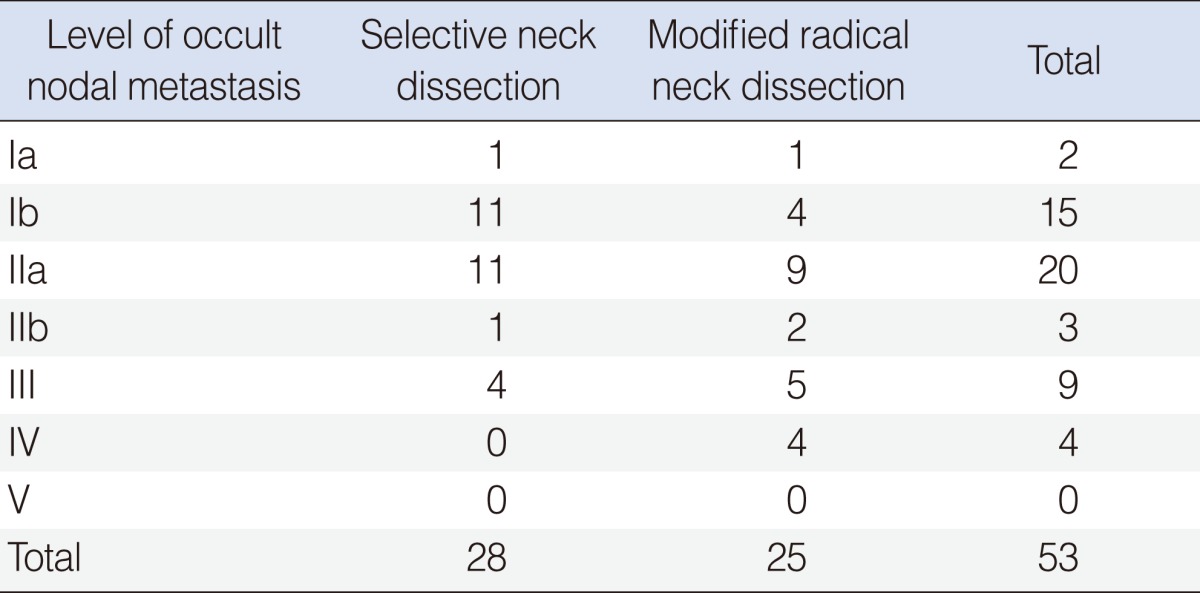
In this study, there was no additional occult metastatic node which we could find by conversion from SND to MRND. In other words, all metastatic levels in conversion MRND group were covered by previously designed SND. level IIa was the most common site for occult neck metastasis in both the SND and MRND groups (n=11 and n=9, respectively) (Table 6). Level Ib was the next most common site (n=11 and n=4, respectively) (Table 6). No occult metastatic node was found in level V (Table 6).
Two or more multilevel nodal metastases were found for 5 cases in SND group and 4 cases in MRND group. There was no statistically significant difference between two groups (Table 7).
When we used the Kaplan Meier survival curve, there was no statistically significant difference of locoregional control rate, overall survival rate and disease specific survival rate between the SND group and the MRND group (P=0.2719, P=0.7596, P=0.2405, respectively) (Fig. 1).
DISCUSSION
In this study, we tried to compare the therapeutic results between two groups; one group received SND for the clinically N negative but final pathologic report after neck dissection was N positive and the other group underwent conversion MRND for the clinically N negative but intraoperatively N positive neck. The Kaplan Meier method didn't show any statistically significant difference of locoregional recurrence rate, overall survival rate and disease specific survival rate between two groups. In other words, the SND itself had the same therapeutic results as that of MRND in this study.
SND has achieved wide acceptance as an elective treatment for the N0 neck [1]. Yet in recent times, SND has been used for the selected patients with clinically positive nodal metastasis [3]. There are many recent studies that have shown that SND, if used in selected patients, has an equivalent disease control rate with that of comprehensive neck dissection for treating node positive patients [4-6]. The development of adjuvant therapy and the increased knowledge about the patterns of tumor spreading to the lymph nodes might be the reason of this change. However, the oncologic safety of using SND for therapeutic purposes is currently unclear.
Yuen et al. [7], designed prospective randomized study of SND versus observation for N0 neck of early tongue carcinoma. In their study, the five-year disease-specific survival rate was 87% for the observation group and was 89% for the SND group. There was no significant statistical difference between two groups. They reported that observation might be an acceptable alternative to elective neck dissection for clinically N0 tongue cancer. In contrast, Jin et al. [8], analyzed the pattern of occult cervical lymph node metastases in 100 consecutive patients with clinically N0 tongue cancer. In their study, the rate of occult cervical lymph node metastasis was 22% and they emphasized the elective and therapeutic role of SND. Gourin et al. [9] analyzed the effect of occult nodal metastases on survival and regional control in patients with head and neck squamous cell carcinoma. They reported that there was a high incidence of occult metastases in clinically node-negative patients which adversely affects survival, regardless of the use of adjuvant therapy.
At our institute, we have applied SND for the clinically N negative neck but whose primary head and neck cancer has a relatively high chance of occult nodal metastases. As for the clinically N positive neck, we have applied comprehensive neck dissection such as RND or MRND. Even though SND was designed initially, if there are suspicious nodes in the operative field, we routinely check the frozen biopsy and convert the neck dissection type from SND to MRND if the result of frozen biopsy is positive. But, we wondered whether the conversion from SND to comprehensive neck dissection was needed or not for clinically node negative but intraoperatively node positive cases. In this 5-year observational study, we concluded that there was no statistically significant difference of locoregional control rate, overall survival rate and disease specific survival rate between SND group and conversion MRND group.
We have used the frozen biopsy result as a determinant for converting the neck dissection type from SND to MRND. Finn et al. [10] reported the accuracy of clinical intraoperative lymph node assessment for metastatic disease in head and neck cancer. They reported the sensitivity of intraoperative lymph node assessment as 56% and the specificity as 70% and reported that the frozen section might not be a good determinate for selection of type of neck dissection. As there were few reports about the accuracy of frozen biopsy result, whether the frozen biopsy is a good determinant for selection of type of neck dissection is still questionable. But with the result in this study, it might not be needed to send the suspicious node for frozen biopsy if preoperative evaluation is node negative as there was no difference of therapeutic result between SND and conversion MRND group.
The pathologic N stage distribution is shown in Table 1. Twenty-three patients were pN1 and 18 patients were pN2b. There was no pN2a patient. This result means that occult nodal metastasis occurred with one or multiple small pathologic nodes rather than with one big node larger than 3 cm in size. If the metastatic node is larger than 3 cm size, then it is easier to find the pathologic node on the preoperative radiologic evaluation.
Level IIa was the most common site of occult nodal metastasis in this study (Table 6). In many other studies, level II was reported as the most common level that metastatic nodes were found [11,12]. But, notably, level Ib was the second common site of occult nodal metastasis. Prevascular, retrovascular, preglandular and retroglandular nodal metastases in level Ib are not easy to find in preoperative radiologic evaluation. Especially, when the primary site is oral tongue, level Ib should be evaluated carefully as occult metastasis to these nodal groups happens frequently.
Extracapsular spread (ECS) of metastatic node is well known important prognostic factor in head and neck cancer. Shaw et al. [13], reported in their study that ECS doubled the incidence of local recurrence and distant metastases, tripled regional failure in oral squamous cell carcinoma. In this study, ECS was positive in all three recurred cases. ECS status should be evaluated especially when there is occult nodal metastasis and when we consider postoperative adjuvant therapy.
The limitation of this study was relatively small number of enrolled cases and postoperative adjuvant therapy which could have an influence on therapeutic results of neck dissection. Further trials with randomized controlled and more enrolled cases are needed to prove the effectiveness of SND for the treatment of occult nodal metastasis.
In conclusion, even for clinically N negative but intraoperatively or postoperatively N positive cases, SND had the same therapeutic results as that of conversion MRND. Our result showed that conversion from SND to MRND is not necessary in the operative field if preoperative evaluation is node negative and unless adhesion or gross tumor invasion to nonlymphatic structures is seen.
Notes
No potential conflict of interest relevant to this article was reported.