Role of Caffeic Acid on Collagen Production in Nasal Polyp-Derived Fibroblasts
Article information
Abstract
Objectives
Caffeic acids are known to have anti-oxidant, anti-inflammatory, immunomodulatory, and tissue reparative effects. The purposes of this study were to determine the effect of caffeic acid on transforming growth factor (TGF) β1-induced myofibroblast differentiation and collagen production, and to determine whether caffeic acid is involved in the antioxidant effect in nasal polyp-derived fibroblasts (NPDFs).
Methods
NPDFs were pretreated with caffeic acid (1-10 µM) for 2 hours and stimulated with TGF-β1 (5 ng/mL) for 24 hours. The expression of α-smooth muscle actin (SMA), collagen types I and III, and Nox4 mRNA was determined by a reverse transcription-polymerase chain reaction, and the expression of α-SMA protein was determined by actin ned by immunofluorescence microscopy. The amount of total soluble collagen production was analyzed by the Sircol collagen dye-binding assay. The reactive oxygen species (ROS) generated by NPDFs were determined using 2',7'-dichlorfluorescein-diacetate. siNox4 was used to determine the effect of Nox4.
Results
The expression of α-SMA and production of collagen were significantly increased following TGF-β1 treatment. In contrast, the level of expression of α-SMA and the level of production of collagen were decreased by pretreatment with caffeic acid. The activation of Nox4 and the subsequent production of ROS were also reduced by pretreatment with caffeic acid. The expression of α-SMA was prevented by inhibition of ROS generation with siNox4.
Conclusion
Caffeic acid may inhibit TGF-β1-induced differentiation of fibroblasts into myofibroblasts and collagen production by regulating ROS.
INTRODUCTION
The pathophysiology of nasal polyp formation is poorly understood. Previous studies have suggested that the proliferation of fibroblasts and differentiation into myofibroblasts has a role in the formation of nasal polyps [1,2]. The myofibroblasts produce extracellular matrix (ECM), such as collagen or fibronectin [3]. Transforming growth factor (TGF) β is a cytokine that stimulates the proliferation of fibroblasts and the differentiation of fibroblasts into myofibroblasts [4]. There is evidence that has shown increased expression of TGF-β1 in nasal polyps compared to normal mucosa and the essential function of TGF-β1 in the growth of nasal polyps [5,6].
A physiologic level of reactive oxygen species (ROS) is crucial for the proper regulation of cell function, such as intracellular signaling, transcription activation, cell proliferation, inflammation, and apoptosis [7]. ROS have been implicated in the pathogenesis of a large number of diseases, including bronchial asthma [8]. ROS are not only generated as by-products in aerobic metabolism, but are also produced by specialized enzymes, such as NADPH oxidases (Noxs) [9].
It has been reported that caffeic acid is a superior antioxidant compared with p-coumaric and ferulic acids in inhibiting low-density lipoprotein oxidation, as well as quenching radicals and singlet oxygen [10,11,12]. However, the effects of caffeic acid on nasal polyp-derived fibroblasts (NPDFs) have not been studied. In this study, the effects of caffeic acid on TGF-β1-induced myofibroblast differentiation and collagen production was determined. We also investigated the under lying molecular mechanisms.
MATERIALS AND METHODS
Reagents
Human recombinant TGF-β1 was obtained from R&D Systems (Minneapolis, MN, USA). Caffeic acid phenethyl ester (CAPE) was purchased from Sigma (St. Louis, MO, USA). CAPE was dissolved in dimethyl sulfoxide.
Induction of fibroblasts and cell culture
To induce fibroblasts from nasal polyps, 6 patients (3 females and 3 males; 29.9±8.2 years of age) were recruited from the Otorhinolaryngology Department of Korea University Guro Hospital. The patients were non-smokers and not treated with anti-allergic agents for at least 2 months. Nasal polyps were obtained during surgical procedures. Fibroblasts were isolated from surgical tissues by enzymatic digestion with collagenase (500 U/mL; Sigma), hyaluronidase (30 U/mL; Sigma), and DNAse (10 U/mL; Sigma). Briefly, following a 2-hour incubation in 5% CO2 at 37℃ in a culture plate, the cells were collected by centrifugation, washed twice, resuspended in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Grand Island, NY, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS), 2-glutamate, 100 µg/mL of penicillin, and 100 µg/mL and streptomycin. The cells were allowed to attach for 4 days. Non-adherent cells were removed by changing the medium. Fibroblast was detached with a trypsin-EDTA solution. After the cells were washed, the cells were resuspended in medium and used for subsequent experiments. The fibroblast purity was >90% and used for NPDFs. Cells were used after passage five. This study was approved by the Institutional Review Institutional Review Board of Korea University College of Medicine (KUGGR-2010-013).
Cytotoxicity assay
The cytotoxic action of CAPE was evaluated by the 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide method. In a 96-well microstate plate, NPDFs (5×103 cells/well) were cultivated in DMEM medium supplemented as described above. CAPE were evaluated at various concentrations (0-10 µM) for 24 hours at 37℃ in a 5% CO2 and 95% humidity atmosphere. After this period, the cells were incubated with 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide (5 mL; Sigma) for 4 hours, and the reaction was interrupted by addition of acidified isopropanol. A fluorescence microplate reader (F2000; Hitachi Ltd., Tokyo, Japan) was used to obtain the results (570 nm). All assays were carried out in triplicate.
Reverse transcription-polymerase chain reaction (RT-PCR)
NPDFs (5×106 cells/mL) were pretreated with CAPE for 2 hours before TGF-β1 (5 ng/mL) stimulation. RT-PCR analysis was performed for α-smooth muscle actin (SMA), collagen types I and III, and Nox4 mRNAs. Total RNA was isolated according to the manufacturer's specifications using an easy-BLUE RNA extraction kit (iNtRON Biotech, Seongnam, Korea). The concentration of total RNA was determined by spectrophotometry. Each sample was reverse-transcribed to cDNA for 60 minutes at 45℃ using a cDNA synthesis kit (Amersham Biosciences Inc., Piscataway, NJ, USA). PCR was performed using the following primers: α-SMA (sense sequence, 5'-GGT GCT GTC TCT CTA TGC CTC TGG A-3'; anti-sense sequence, 5'-CCC ATC AGG CAA CTC GAT ACT CTT C-3', 322 bp); collagen type I (sense sequence, 5'-GTC TTC CTG GCC CCT CTG GTG-3'; anti-sense sequence, 5'-TCG CCC TGT TCG CCT GTC TCA-3', 391 bp); collagen type III (sense sequence, 5'-CTG GAG GAG CTG GCT CGC CAA CGA AG-3'; anti-sense sequence, 5'-GTG ATC ATG AGG AAT AGC ACC ACC ACC ATG-3', 250 bp); Nox4 (sense sequence, 5'-CTG GAG GAG CTG GCT CGC CAA CGA AG-3'; anti-sense sequence, 5'-GTG ATC ATG AGG AAT AGC ACC ACC ACC ATG CAG-3', 516 bp); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sense sequence, 5'-GTG GAT ATT GTT GCC ATC AAT GAC C-3'; anti-sense sequence, 5'-GCC CCA GCC TTC TTC ATG GTG GT-3', 271 bp) were purchased from Bioneer (Daejeon, Korea). The GAPDH was used to verify that equal amounts of RNA were used for RT-PCR amplification from different experimental conditions. Products were electrophoresed on a 1.5% agarose gel and visualized by staining with ethidium bromide. The gels were certificated using a Kodak DC 290 digital camera (Eastman Kodak, Rochester, NY, USA) and digitized using UN-SCAN-IT software (Silk Scientific, Orem, UT, USA).
Immunocytochemical staining of α-SMA
NPDFs (2×103 cells/mL) were plated on eight-well chamber slides (Nalge Nunc International, Rochester, NY, USA). Fibroblasts were pretreated with CAPE before TGF-β1 stimulation for 24 hours. Then, the cells were fixed in phosphate buffered saline (PBS) containing 4% paraformaldehyde for 30 minutes, blocked with 3% bovine serum albumin, and incubated with an monoclonal anti-α-SMA antibody (1:200) for 2 hours, washed 2 times with PBS. Cells were then incubated in goat anti-mouse IgG Alexa Fluor 488 (Invitrogen) at 1:200 for 1 hour. Then, the cells were mounted in Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) with 4',6-diamidino-2-phenylindole (DAPI). Cells were observed on a fluorescence microscope.
Collagen measurements
Total soluble collagen in cell culture supernatants is quantified using the Sircol collagen assay (Biocolor, Belfast, UK). For these experiments, confluent cells in 25 cm2 culture dishes are incubated for 24 hours with 1 mL DMEM-5% FBS. One milliliter of Sirius red dye, an anionic dye that reacts specifically with basic side chain groups of collagens under assay conditions, was added to 400 µL of supernatant and incubated with gentle rotation for 30 minutes at room temperature. After centrifugation at 12,000 g for 10 minutes, the collagen-bound dye was redissolved with 1 mL of 0.5 M NaOH, and absorbance at 540 nm was measured by enzyme-linked immunosorbent assay (MRX; Dynex, Chantilly, VA, USA). The absorbance was directly proportional to the amount of newly formed collagen in the cell culture supernatant.
Assay of intracellular ROS
The production of intracellular ROS was also determined by fluorescent microscope using a fluorescent probe, 2', 7'-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes Inc., OR, USA). DCFH-DA diffuses through the cell membrane readily and is enzymatically hydrolyzed by intracellular esterases to non-fluorescent DCFH, which is then rapidly oxidized to highly fluorescent DCFH in the presence of ROS. The stock DCFH-DA (2 mM) was prepared in absolute ethanol and kept at -70℃ in the dark. Cells collected from a 12-well plate using 0.5% trypsin/EDTA were washed twice with PBS prior to the analysis. Cells were incubated with 20 µM DCFH-DA for 1 hour, then with maximum concentrations of CAPE for another 2 hours prior to treatment with TGF-β1 for 24 hours. After washing 3 times with PBS, cells were examined with fluorescent microscope (excitation [488 nm] and emission [520 nm], Olympus IX71; Tokyo, Japan).
Transfection with small interference RNA
For Nox4 RNA interference, a siRNA specific for human NOX4 (siNox4 sense, 5'-ACU GAG GUA CAG CUG GAU GUU-3'; and anti-sense, 3'-CAU CCA GCU GUA CCU CAG UUU) was used. As a control, the universal negative control siRNA (siCont; Invitrogen) was used. Individual siRNAs (100 nmol/L), lipofectamine, and Opti-MEM medium were mixed and incubated at room temperature for 30 minutes. siRNA-lipofectamine complexes were added to cells for 48 hours, after which siRNA-lipofectamine complexes were removed and cells were washed and placed in serum-free medium for 24 hours. Subsequently, cells were treated with or without TGF-β1 for the indicated time and harvested for RNA extraction.
Statistical analysis
Data were described as the mean±SE. Statistical analysis was performed using the Student t-test or analysis of variance (ANOVA), as appropriate. P<0.05 was considered statistically significant.
RESULTS
The effect of CAPE on α-SMA expression in TGF-β1-induced NPDFs
The cytotoxicity of CAPE on fibroblasts was examined using a 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide assay. CAPE (up to 10 µM) showed no cytotoxic effects for 48 hours (Fig. 1). Next, cells were pretreated with CAPE (1 and 5 µM) and stimulated by TGF-β1 for 24 hours. The level of α-SMA mRNA expression was determined by RT-PCR. The maximum dimethyl sulfoxide concentration present in the final working dilutions was <0.1%. TGF-β1 significantly increased the level of α-SMA mRNA expression. Pretreatment with CAPE significantly inhibited TGF-β1-induced α-SMA mRNA expression in 5 µM (Fig. 2A, B).

Cytotoxicity test using 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl-2H-tetrazolium bromide assay at various concentrations of caffeic acid phenethyl ester (CAPE) in nasal polyp-derived fibroblasts. Values are expressed as the mean±SE of 6 separate experiments.
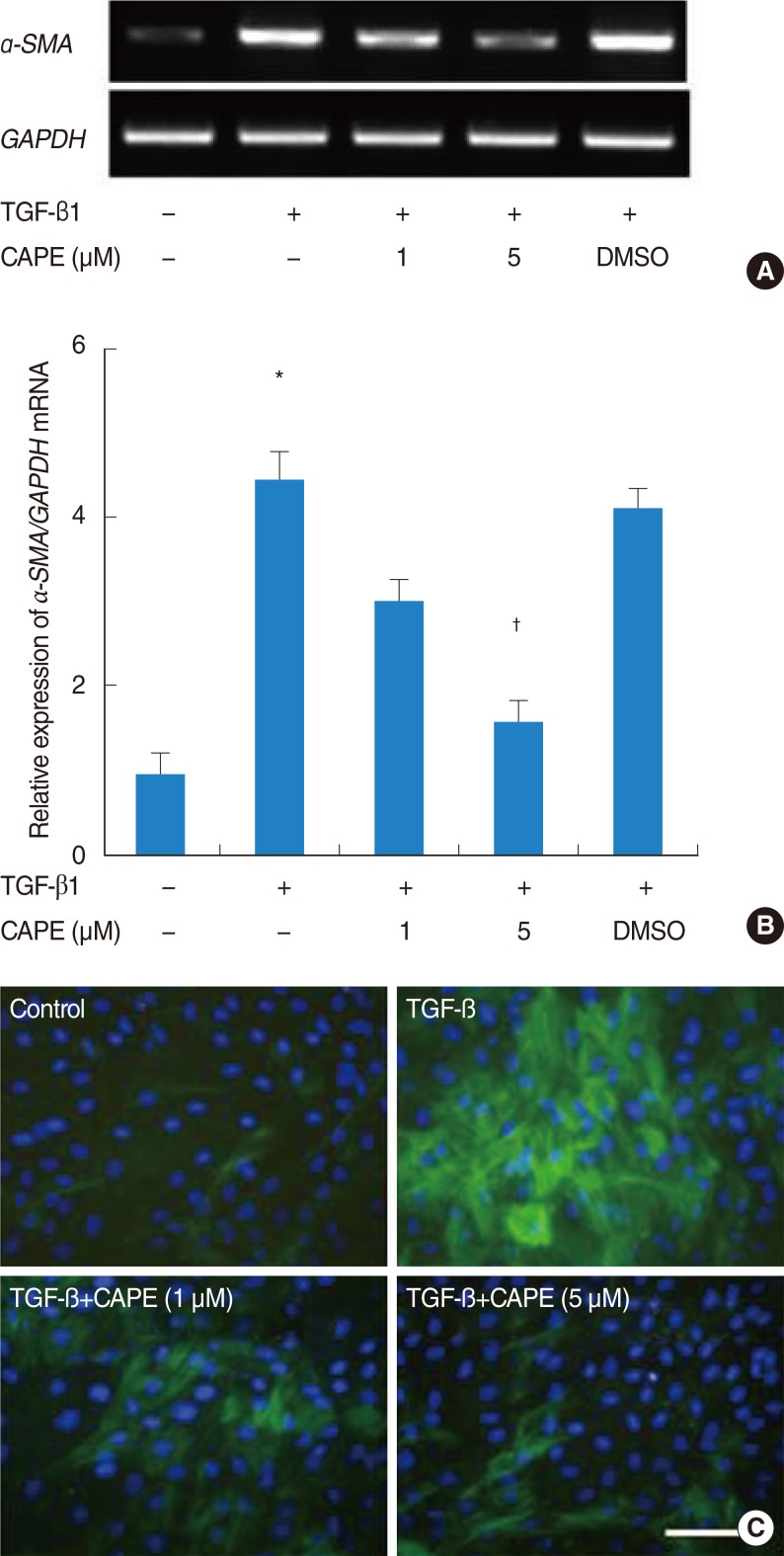
Effects of caffeic acid phenethyl ester (CAPE) on expression of α-smooth muscle actin (SMA) mRNA in transforming growth factor (TGF) β1-induced nasal polyp fibroblasts, as determined by RT-PCR (A) and densitometric analysis (B). The expression of GAPDH is shown as an internal control. Cells were also treated with 0.1% demethyl sulfoxide (DMSO) in the absence of CAPE. Values are expressed as the mean±standard error of six separate experiments. Effects of CAPE on α-SMA protein expression in TGF-β1-induced nasal polyp-derived fibroblasts (NPDFs), as determined by immunofluorescent staining (C). NPDFs were treated with media alone. NPDFs induced by TGF-β1. NPDFs induced by TGF-β1 were pretreated with CAPE (1 µM) and CAPE (5 µM). Original magnification (×200). *P<0.05 vs. control, †P<0.05 vs. TGF-β1 alone. Scale bar=100 µm.
To determine the inhibitory effect of CAPE in TGF-β1-induced myofibroblast differentiation (α-SMA protein), NPDFs were pretreated with CAPE and stimulated them with TGF-β1 for 48 hours. The addition of CAPE decreased the number of α-SMA protein-positive cells and it was significant in 5 µM (Fig. 2C).
The effect of CAPE on collagen production in TGF-β1-induced NPDFs
To determine the inhibitory effect of CAPE in TGF-β1-induced collagen types I and III mRNA expression, cells were treated with CAPE for 2 hours before stimulating the cells with TGF-β1 for 24 hours. The expression of collagen types I and III mRNA were increased by stimulation with TGF-β1, and were notably decreased by pretreatment with CAPE in 5 µM (Fig. 3A, B).
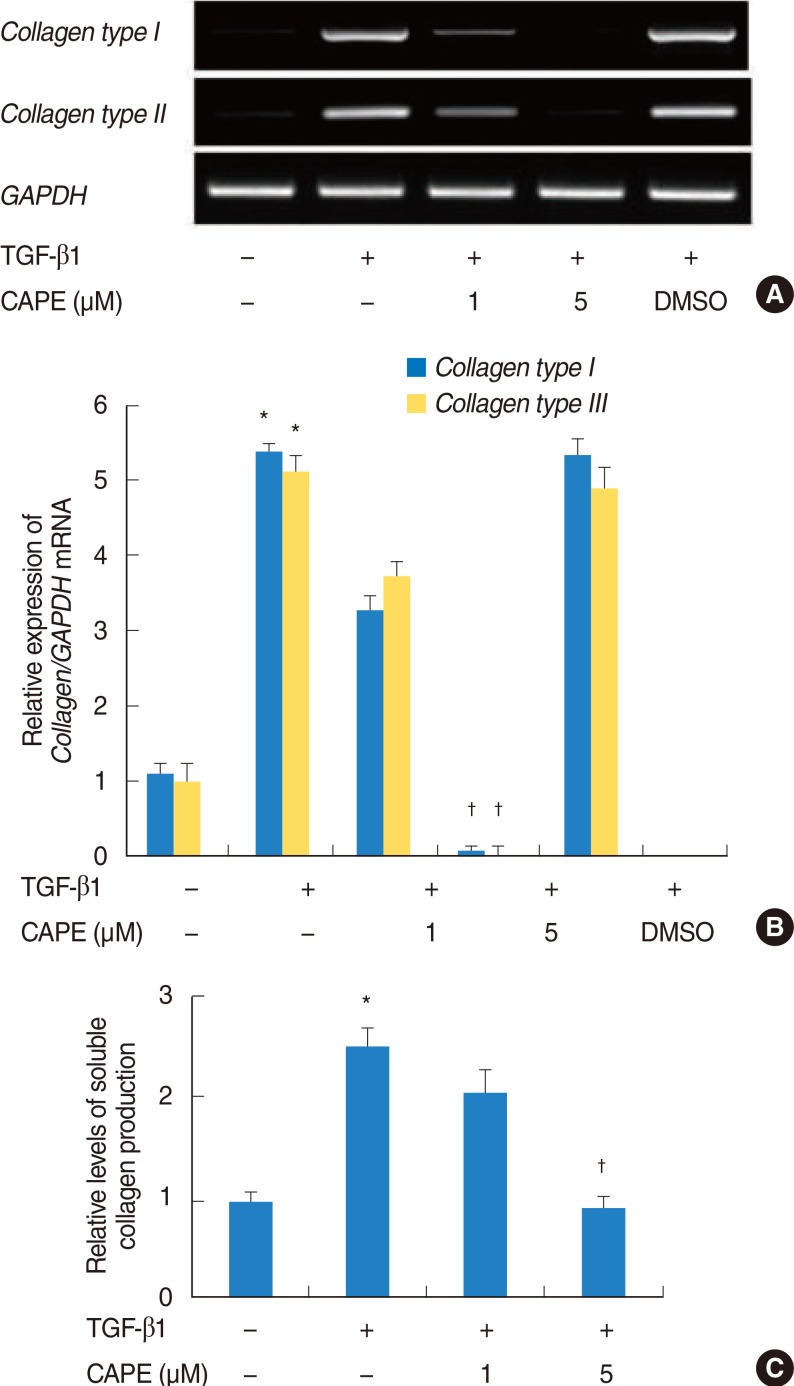
Effects of caffeic acid phenethyl ester (CAPE) on the expression of collagen types I and III mRNA in transforming growth factor (TGF) β1-induced NPDFs, as determined by RT-PCR (A, representative of six independent experiments) and densitometric analysis (B). The expression of GAPDH is shown as an internal control. Cells were also treated with 0.1% demethyl sulfoxide (DMSO) in the absence of CAPE. The effects of CAPE on total soluble collagen production in TGF-β1-induced nasal polyp-derived fibroblasts were determined by a Sircol collagen dye-binding assay (C). Values are expressed as the mean±SE. *P<0.05 vs. control, †P<0.05 vs. TGF-β1 alone.
To determine the inhibitory effect of CAPE in TGF-β1-induced soluble collagen production, cells were pretreated with CAPE and stimulated with TGF-β1 for 48 hours. The addition of CAPE noticeably reduced TGF-β1-induced soluble collagen production in 5 µM (Fig. 3C).
The effect of CAPE on intracellular ROS generation in TGF-β1-induced NPDFs
The anti-oxidant effect of CAPE in TGF-β1-induced intracellular ROS production was also confirmed on fluorescent microscopy. Pretreatment with CAPE inhibited TGF-β1-induced intracellular ROS production compared with TGF-β1 treatment alone (Fig. 4).

Fluorescent microscopic finding of intracellular reactive oxygen species (ROS), original magnification (×200). Control fibroblasts expressed small amount of ROS production. After treatment with transforming growth factor (TGF) β1, ROS markedly increased. Pretreatment with caffeic acid phenethyl ester (CAPE, 1 and 5 µM) strongly inhibited induced production of ROS in dose-dependent manner. (A) No treatment. (B) TGF-β1 (5 ng/mL) alone. (C) TGF-β1 and CAPE (1 µM). (D) TGF-β1 and CAPE (5 µM). Scale bar=100 µm.
Effect of CAPE on Nox4 expression in TGF-β1-induced NPDFs
Because TGF-β1 increased ROS production in NPDFs, we hypothesized that ROS production is mediated by increased expression of Noxs. Previously, we showed that up-regulation of Nox4 may be important for TGF-β1 effects on nasal fibroblasts (data not shown). To determine the inhibitory effect of CAPE in TGF-β1-induced Nox4 mRNA expression, cells were pretreated with CAPE and stimulated with TGF-β1 for 12 hours. The expression of Nox4 mRNA was considerably decreased by pretreatment with CAPE in 5 µM (Fig. 5A, B).
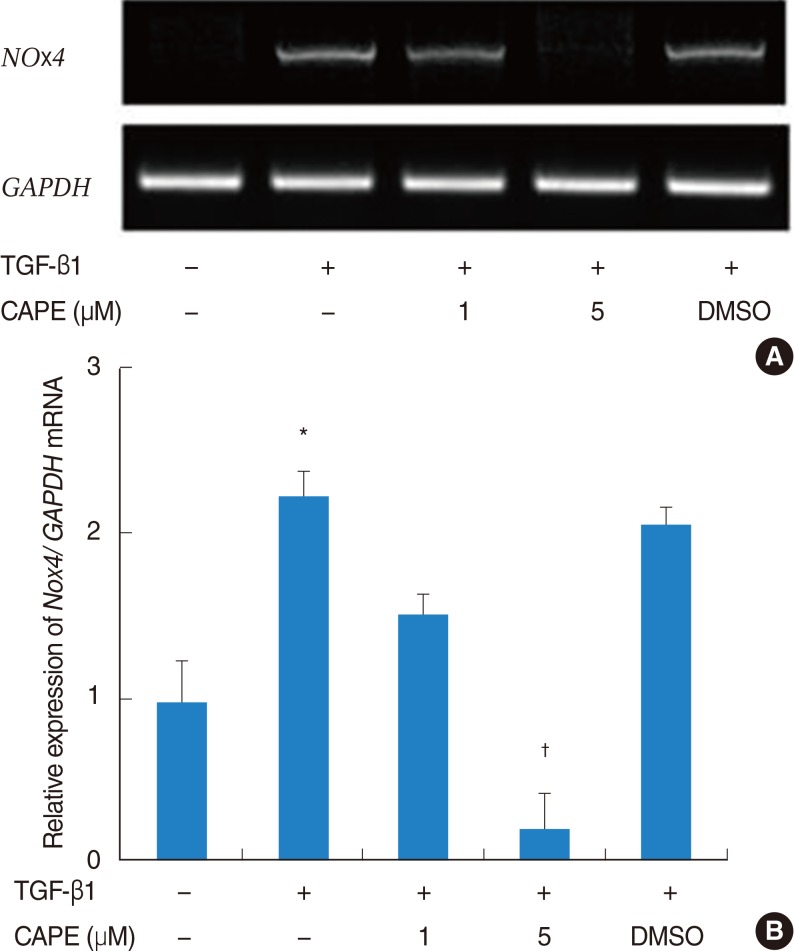
Effect of caffeic acid phenethyl ester (CAPE) on Nox4 mRNA expression in transforming growth factor (TGF) β1-induced nasal polyp-derived fibroblasts, as determined by RT-PCR (A, representative of six independent experiments) and densitometric analysis (B). The expression of GAPDH is shown as an internal control. Cells were also treated with 0.1% demethyl sulfoxide (DMSO) in the absence of CAPE. Values are presented as the mean±SE. *P<0.05 vs. control, †P<0.05 vs. TGF-β1 alone.
Nox4 is required for α-SMA expression in TGF-β1-induced NPDFs
Additional studies were performed to determine whether or not Nox4 is necessary for α-SMA expression in TGF-β1-induced NPDFs. We down-regulated the expression of Nox4 mRNA using transfection with small interference oligonucleotide RNA directed against Nox4 (siNox4). We already confirmed that siNox4 decreases the level of Nox4 mRNA in our previous study [13]. AntiOn transfection with siNox4, TGF-β1-induced expression of Nox4 mRNA was reduced. To assess whether Nox4 is required for α-SMA expression, α-SMA at the mRNA level was quantified after transfection of cells with siNox4. The α-SMA mRNA was markedly reduced by siRNA against Nox4 (Fig. 6).

Nox4 mediates transforming growth factor (TGF) β1-induced α-smooth muscle actin (SMA) mRNA in nasal polyp-derived fibroblasts. Cells were transfected with small interfering RNA (siCont or siNox4) and stimulated for 12 hours with TGF-β1, and α-SMA mRNA was semi-quantified by RT-PCR (A). Mean±SE of densitometric data (B). As a control, the universal negative control siRNA (siCont) were employed. *P<0.05 vs. control, †P<0.05 vs. TGF-β1 alone.
DISCUSSION
In the present study, we showed that CAPE inhibits the expression of α-SMA, an indicator of myofibroblast differentiation and collagen production in NPDFs, and that CAPE inhibits ROS production and Nox4 expression. We confirmed that on transfection with siNox4, TGF-β1-induced expression of Nox4 mRNA was reduced, implicating that inhibition of phenotypic change of NPDFs by CAPE was mediated by the antioxidant effect.
CAPE is an active phenolic compound that is present in propolis, which is the generic name of a resinous product derived from conifer bark and carried by honeybees to their hives [14]. CAPE has been shown to have several interesting biological properties, including antioxidant, anti-inflammatory, anti-viral, immunostimulatory, anti-angiogenic, anti-invasive, anti-metastatic, and carcinostatic properties [15]. Although CAPE has been shown to have antioxidant, anti-inflammatory, immunomodulatory, and anti-mutagenic effects, the effects on parameters related with nasal polyps have not been investigated.
Although the etiology of nasal polyps and the pathophysiologic mechanisms leading to the formation of nasal polyps are poorly understood, a number of studies have suggested that the differentiation of fibroblasts into myofibroblasts and ECM accumulation are key processes [1]. TGF-β1, which is highly expressed in nasal polyp tissues, is thought to be involved in the structural modifications that characterize nasal polyp formation [6]. In our previous study, we confirmed that TGF-β1 significantly increased the expression of α-SMA, as well as the production of collagen types I and III in NPDFs, and Nox4 and ROS play an important role in phenotypic change and ECM formation of TGF-β1-induced NPDFs [16].
There are several studies regarding the association between oxidative stress and nasal polyps. Dagli et al. [17] investigated the role of free radicals and antioxidants in nasal polyps and suggested that the levels of antioxidants were decreased and the levels of oxidants were increased. Cheng et al. [18] demonstrated that the mean level of tissue chemiluminescence in nasal polyps was significantly higher than control specimens, and the expression of superoxide dismutase 1 and 3 was higher in nasal polyp tissues. However, these studies focused on mucosal damage by ROS, while we focused on the role of ROS in signal mediators in TGF-β1-induced NPDFs.
In summary, we have identified and demonstrated evidence that CAPE has inhibitory effects on TGF-β1-induced α-SMA expression of NPDFs. CAPE also inhibited ROS production and Nox4 expression at the same concentration. These findings support the notion that CAPE has antioxidant effects that are associated with the modulation of myofibroblast differentiation which occurs in the pathogenesis of nasal polyps. Further studies are necessary to determine whether CAPE shows inhibitory effects on the formation of nasal polyps as occurs in vivo.
In conclusion, CAPE inhibits myofibroblast differentiation of TGF-β1-activated NPDFs and collagen production; the effects of CAPE on Nox4 and ROS are involved in the process. These results show that CAPE may play an inhibitory role in the development of nasal polyps.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Projects, Minister for Health, Welfare & Family Affairs, Republic of Korea (A090084).
Notes
No potential conflict of interest relevant to this article was reported.