Loss of Heterozygosities in Five Tumor Suppressor Genes (FHIT Gene, p16, pRb, E-Cadherin and p53) in Thyroid Tumors
Article information
Abstract
Objectives
To evaluate the loss of heterozygosities (LOH) of chromosomes 3p14 (FHIT gene), 9p21 (p16), 13q21 (pRb), 6q22 (E-cadherin) and 17p13 (p53) in various thyroid tumors.
Methods
Eighty thyroid tumor cases (20 follicular adenomas, 10 follicular carcinomas, and 50 papillary carcinomas) have been analyzed for the presence of LOH in chromosomes 3p14, 9p21, 13q21, 6q22, and 17p13 allelic loss, using microsatellite markers and DNA obtained from formalin-fixed paraffin-embedded archival tissues.
Results
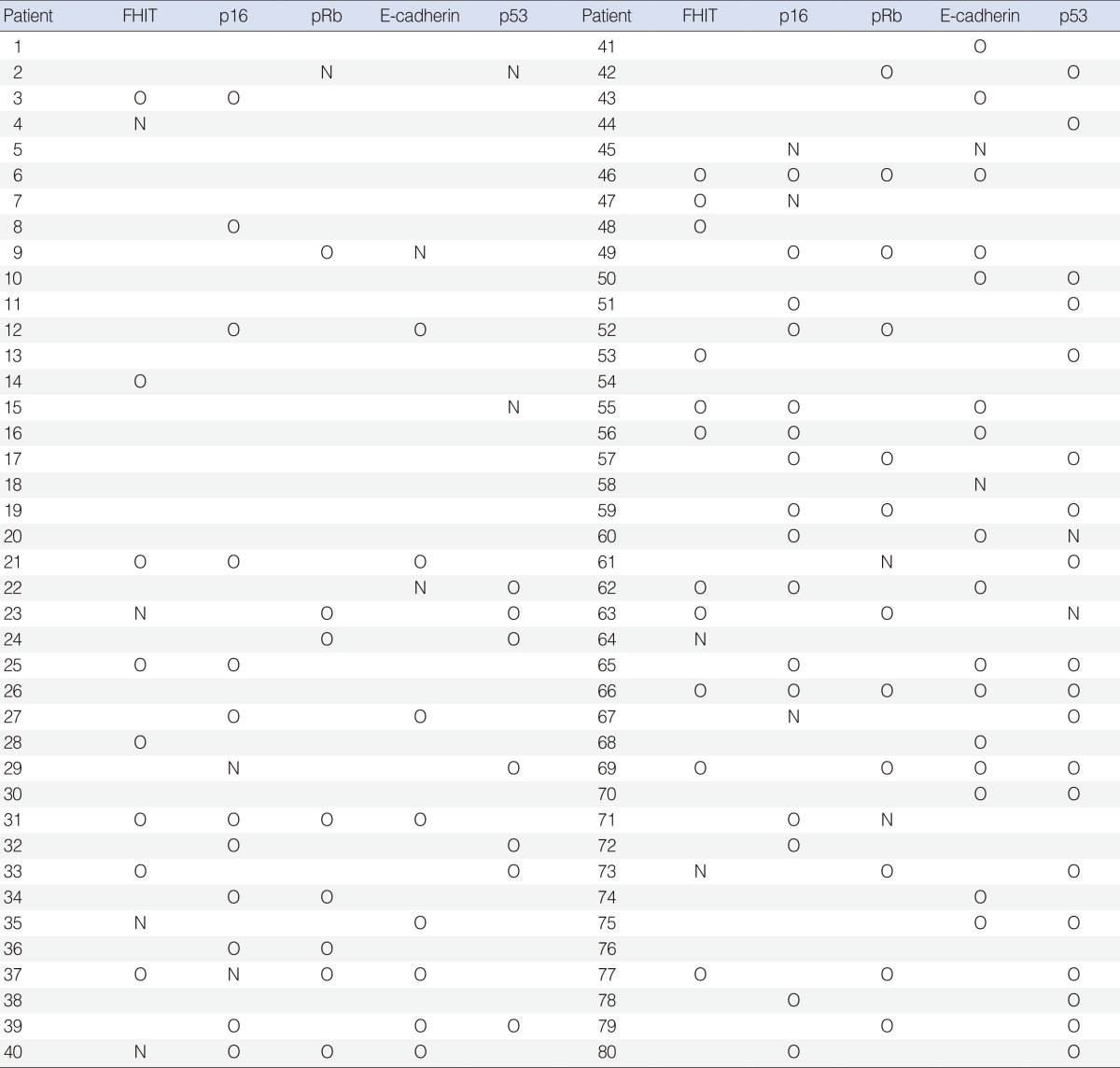
LOH on 3p14 was found in 10.5%, 33.3%, and 30.4% of follicular adenomas, follicular carcinomas, and papillary carcinomas, respectively. LOH on 9p21 was detected in 6%, 44.4%, and 47.8%, respectively. LOH on pRb gene was found in 5.3%, 20.0%, and 35.4%, respectively. LOH on E-cadherin gene was found in 5.3%, 22.2%, and 43.8%, respectively. LOH on 17p13 was detected in 0%, 40%, and 45.8%, respectively. LOH in FHIT gene, p16, pRb, E-cadherin, and p53 genes were more frequently identified in follicular carcinoma and papillary carcinoma than in follicular adenoma.
Conclusion
LOH results of the five tumor suppressor genes (FHIT gene, p16, pRb, E-cadherin, and p53) showed statistical differences between benign tumor and malignant tumor. Among papillary carcinoma, LOH in p16, E-cadherin and p53 genes well correlated with poorly differentiated grade, and LOH of E-cadherin was associated with lymph node metastasis.
INTRODUCTION
Thyroid cancer is one of major malignancies in young adult patients and recently shows an increasing tendency in incidence in Korea. Most thyroid nodular lesions are asymptomatic and their early detection is quite difficult. Recently, much effort to elucidate genetic events as prognostic factors of thyroid nodular lesions have been made. Microsatellite alteration (MSA) such as loss of heterozygosity (LOH) has been reported as a novel mechanism of carcinogenesis and as a useful prognostic factor in many malignant tumors. LOH, the loss of a functional allele at a heterozygous locus, is known to be related with allelic loss of various tumor suppressor genes (TSGs). Many TSGs (such as FHIT gene, p16, pRb, E-cadherin, p53, etc.) are known to be lost or mutated in a variety of thyroid tumors [1-6]. FHIT is a candidate TSG, and LOH in FHIT gene have been reported in many types of human cancers [1,7,8]. The p16 protein functions as a tumor suppressor by binding to the cyclin D1 CDK4/CDK6 complex, thereby preventing phosphorylation of the pRb protein [9]. Alterations in p16INK4A gene prevents blocking the cell from entering the S phase at the late G1 checkpoint, thus resulting in tumorigenesis [10]. The retinoblastoma (Rb) gene was the first TSG to be discovered, and it is known to be associated in many malignancies including leukemia, malignant melanoma, lung cancer, bladder cancer, esophageal cancer, and pancreatic cancer, etc [5]. E-cadherin is a transmembrane protein engaged in cell adhesion, the disruption of which results in carcinogenesis, and it is thought that alterations in cell-cell adhesion proteins influence later events in carcinogenesis such as invasion and metastasis [11]. p53 is a 53kD nuclear protein, highly conserved in vertebrates, which is believed to regulate entry into and progression through the normal cell cycle. Elevated p53 expression has been described in a number of human tumors including carcinoma of the breast, colorectum, and lung [12]. Evaluation of these genetic alterations may certainly translate into clinical benefits.
The purpose of this study is to investigate the LOH in five major TSGs (FHIT gene, p16, pRb, E-cadherin, and p53) in 80 cases of surgically resected thyroid tumors, and to correlate the results with various clinicopathological factors in 50 papillary thyroid carcinomas.
MATERIALS AND METHODS
Eighty patients with thyroid tumors, who underwent surgery in a tertiary medical center (Ilsong Memorial Institute of Head and Neck Cancer, Hallym University College of Medicine, Korea) from September 2008 to November 2010 were consecutively selected, which was approved by the resident Institutional Review Board. Of the 80 cases, 37 were men and the overall mean age was 51.2 years (range 27 to 81 years). The cases consisted of 50 papillary carcinomas, 10 follicular carcinomas and 20 follicular adenomas.
LOH detection was carried out sequentially by DNA preparation, PCR, and agarose gel electrophoresis. We extracted DNA from 10% formalin-fixed paraffin-embedded thyroid tissues and matched healthy tissues (preferentially cancer-negative lymph node, obtained from a different tissue block containing only normal tissue). Hematoxylin and eosin (H&E) stained specimens were soaked in 2% glycerol for 2 minutes, then dissected under a microscope using 25-gauge needles and a microdissector. Extracted tissues were placed in individual 1.5-mL Eppendorf tubes filled with buffer solution (100 mM Tris-HCl [pH 8.0], 1% Tween-20, 0.1 mg/mL proteinase K) and were incubated in a 52℃ water tank for 2 days. Then, 1 µL of DNA from these specimens was used as a template for PCR. The presence of LOH in chromosomes 3p14 (fragile histidine triad, FHIT), 9p21 (p16), 13q21 (pRb), 6q22 (E-cadherin), and 17p13 (p53) were analyzed using PCR with 10 microsatellite markers (two markers per gene) (3p14: D3S1300 and D3S1067; 9p21: D9S104 and D9S162; 13q21: D13S118 and D13S153; 6q22: D16S419 and D16S3106; 17p13: TP53 and D17S796). PCR products were electrophoresed on 2% agarose gels with formamide loading dye (95% formamide, 20 mM EDTA, 10 mM NaOH, 0.05% bromophenol blue, 0.05% xylene cyanol), silver-stained, and analyzed for heterozygosity. The gels were evaluated by visual inspection by two pathologists. LOH was considered to be present when a clear reduction of one allele was evident by visual inspection, i.e., presence of an absent allele in the carcinoma or a tissue that showed less than 50% density as the corresponding normal tissue in heterozygotes (Fig. 1). For each genetic alteration, cases with homozygosity or with insufficient amplification were excluded as noninformative. Disease-free survival was measured from the date of surgical resection to the date of diagnosis of recurrence, the date of death, or the date of the final follow-up visit.

Representative examples of cases with loss of heterozygosity (LOH) in FHIT, p16, pRb, E-cadherin, and p53 gene of thyroid tumors. Each LOH detection was conducted using the microsatellite markers (D3S1300, D9S104, D13S118, D16S419, and TP53, respectively). Each arrow shows the remarkable band loss, while the left lane indicates normal (N) and the right lane indicates tumor (T).
Patients with papillary carcinoma were stratified according to their clinicopathologic factors and the presence or absence of LOH for each gene. According to the pathological differentiation, papillary carcinomas were further classified into well or poorly differentiated carcinoma. Statistical analysis was performed using the χ2 test, Fisher exact test, and Cox regression analysis. The Kaplan-Meier test was used for survival analysis. P<0.05 were considered statistically significant.
RESULTS
Detection of LOH in TSGs in thyroid tumors
LOH was detected in all 10 microsatellite markers tested. Among the three different thyroid tumors, five cases of follicular adenoma (25%), eight cases of follicular carcinoma (80%) and 44 cases of papillary carcinoma (88%) had at least one LOH (Table 1). When all five TSGs (FHIT gene, p16, pRb, E-cadherin, and p53) were considered, significant difference in LOH detection rate between adenomas and carcinomas were found (P=0.002).
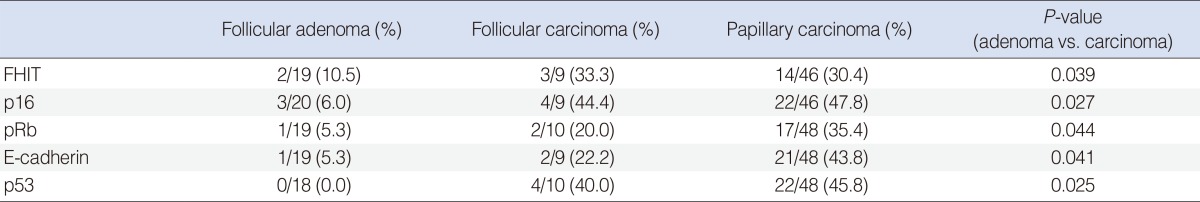
For the FHIT gene, we were able to evaluate 19, 9, and 46 cases for follicular adenoma, follicular carcinoma and papillary carcinoma, respectively. Noninformative cases of each tumor were 1, 1, and 4 cases. Among the informative cases, LOH was detected in 2 cases (10.5%), 3 cases (33.3%), and 14 cases (30.4%), respectively (Table 2), and no significant correlation between tumor types were noticed. But, when we analyzed by adenoma (follicular adenoma) versus carcinoma (follicular carcinoma and papillary carcinoma), LOH was detected significantly higher in carcinomas than in adenomas (P=0.039). For the TSG p16, informative cases of follicular adenoma, follicular carcinoma and papillary carcinoma were 20, 9, and 46 cases, respectively. Among these, LOH was detected in 3 cases (6%), 4 cases (44.4%), and 22 cases (47.8%), respectively, and carcinomas showed significant higher LOH in p16 than adenomas (P=0.027). For the pRb gene, we were able to evaluate 19, 10, and 48 cases for follicular adenoma, follicular carcinoma and papillary carcinoma, respectively. Among these informative cases, LOH was detected in 1 case (5.3%), 2 cases (20%), and 17 cases (35.4%), respectively, and malignant tumors showed significant higher LOH in pRb than benign tumors (P=0.044). For the E-cadherin gene, we were able to evaluate 19, 9, and 48 cases for follicular adenoma, follicular carcinoma and papillary carcinoma, respectively. Among these, LOH was detected in 1 case (5.3%), 2 cases (22.2%), and 21 cases (43.8%), respectively, and malignant tumors showed significant higher LOH in E-cadherin than benign tumors (P=0.041). In cases with p53 gene, informative cases were 18, 10, and 48 cases for follicular adenoma, follicular carcinoma and papillary carcinoma, respectively. Among these, LOH was detected in 0, 4 cases (40%), and 22 cases (45.8%), respectively, and carcinomas had significant higher LOH in p53 than adenomas (P=0.025).
Correlations of LOH with clinicopathologic parameters in papillary carcinoma
For papillary carcinoma cases, we observed genetic alterations and their associations between clinical and pathological parameters, as summarized in Table 3. In tumors with size 2.0 cm or more, the LOH in FHIT gene were detected in 6 cases (33.3%), and 8 cases (25.0%) were detected in tumors less than 2.0 cm, revealing no significant correlation between FHIT LOH and the size of the carcinoma. Well differentiated carcinomas had LOH in FHIT gene in 9 cases (25.7%), and poorly differentiated carcinomas in 5 cases (33.3%), revealing no significant correlation in cancer differentiation. Five cases (33.3%) had lymphatic emboli, 9 cases had none (25.7%), which revealed no significant correlation between the LOH in FHIT gene and the presence of lymphatic emboli. Six cases (33.3%) had extrathyroid invasion and 8 cases (25.0%) had none, which showed no significant correlation. Tumors which had lymph node metastasis were 7 cases (30.4%), which had none, were 7 cases (25.9%), showing no significant difference.
Loss of heterozygosity at each chromosomes according to the clinicopathological factors in papillary carcinoma
In cases with the p16 gene, LOHs were found in 9 cases (50.0%) in papillary carcinomas with size 2.0 cm or more, and 13 cases (40.6%) in carcinomas less than 2.0 cm in size, revealing no significant correlation between LOH and the size of the carcinoma. Well differentiated carcinomas had LOH in p16 gene in 13 cases (37.1%), and poorly differentiated carcinomas in 9 cases (60.0%). These showed that poorly differentiated papillary carcinomas had significantly higher rate of LOH in p16, than well differentiated carcinomas (P=0.044). The presence of lymphatic emboli, extrathyroid invasion or lymph node metastasis were not significantly associated with the LOH in p16.
For the pRb gene, the size of the carcinoma were not associated with the detection rate of the LOH. LOH were found in 10 cases (28.6%) of well differentiated carcinomas, and 7 cases (46.7%) of poorly differentiated carcinomas. Although higher detection rates were seen in poorly differentiated carcinomas, no statistically significant correlation was found. Lymphatic emboli, extrathyroid invasion or lymph node metastasis were not significantly associated with the LOH in pRb.
For the E-cadherin gene, carcinomas with size 2.0 cm or more showed higher rate of LOH than smaller size carcinomas (50.0% vs. 37.5%), but without statistical significance. Well differentiated carcinomas had LOH in 12 cases (34.3%), and poorly differentiated carcinomas in 9 cases (60.0%), which revealed higher detection rate in poorly differentiated carcinomas with significance (P=0.048). The detection rate was higher in the cases with the presence of lymphatic emboli or extrathyroid invasion, but without statistical significance. The tumors with lymph node metastasis had higher rate of LOH in E-cadherin compared to those without lymph node metastasis (60.9% vs. 25.9%; P=0.025).
The size of the carcinoma and the presence of lymphatic emboli were not associated with the detection rate of LOH in p53 gene. Poorly differentiated carcinomas showed higher rate of LOH than well differentiated carcinomas (66.7% vs. 34.3%), with significance (P=0.035). The presence of LOH were higher in carcinomas with extrathyroid invasion or lymph node metastasis, but neither showed statistical significance.
We did not observe any associations between LOH of these five TSGs and sex nor age, in papillary carcinoma. Moreover, disease-free survival was not correlated with the presence of LOH at any of the different chromosomal regions, in this study.
DISCUSSION
Recent advances in radiologic and histopathologic diagnosis enabled more accurate detection of the tumor, but genetic events associated with thyroid tumorigenesis are still poorly defined. Furthermore, the impacts of genetic events on prognosis and therapeutic options for patients with thyroid tumors have not been fully established. This study analyzed LOH in several well-characterized TSGs, i.e., FHIT, p16, pRb, E-cadherin, and p53 in follicular adenoma, follicular carcinoma and papillary carcinoma, and the relationship of LOH and major clinicopathological factors of papillary carcinoma, to assess the possibility of these TSGs as prognostic factors of thyroid cancers.
We observed a significant higher detection of LOH in the five TSGs (FHTI gene, p16, pRb, E-cadherin, and p53) in carcinomas than in adenomas, as follicular adenoma showed low detection rate. Studies related to FHIT gene in thyroid tumors are rare, but our results corresponded to those of Zou et al. [1]'s, which showed difference of detection rate between follicular adenoma and follicular carcinoma. Similar results were found for the p16 gene in our study, the detection rate of LOH was lower in follicular adenoma, and significant difference was noted between adenomas and carcinomas. This also correlated with studies reported by Roque et al. [2] which showed higher incidence of LOH in follicular carcinoma than in follicular adenoma, and studies reported by Kitamura et al. [3] which showed high detection rate of LOH in papillary carcinoma. Soares et al. [13] reported that the LOH in E-cadherin were rarely found in papillary carcinoma. However, our results showed that the larger size of the cancer, the presence of lymphatic emboli or extrathyroidal invasion, had tendency to have more LOH in E-cadherin gene. Moreover, poorly differentiated carcinoma or the presence of lymph node metastasis were associated with higher detection rate of LOH with statistical significance. This was consistent with the report of Naito et al. [6]'s, which noted that the loss of E-cadherin expression may be involved in regional lymph node metastasis and in malignant potential of thyroid neoplasms. But, further studies with a larger number of cases will be needed in this gene, since some other studies reported no association [13], and in some, decreased expression of E-cadherin was reported as a significant predictor of lymph node metastases [14]. For the p53 gene, LOH was not found in follicular adenoma in this study. However, in follicular carcinoma and in papillary carcinoma, the detection rates were 40% and 45.8%, respectively. This showed significant differences between benign and malignant tumors, which had coincidence with other studies [4].
Previous authors have hypothesized that the accumulation of several gene abnormalities results in the formation of cancers, with the quality and quantity of these abnormalities influencing the degree of malignancy [15]. We could not detect a specific higher LOH related to the malignant potential of thyroid neoplasm, nor a correlation of survivals with the LOH, but it is considered that each chromosomes and their LOH independently contributes to the tumorigenesis of the thyroid gland, since we found that the five TSGs' LOH detection rate were significantly higher in malignant tumors than in benign tumors. Although, the detection rate of LOH in papillary carcinomas did not show correlation with sex, age, tumor size, presence of lymphatic emboli nor extrathyroid invasion, the LOH of p16, E-cadherin, and p53 genes well correlated with poorly differentiated grade, and LOH of E-cadherin was associated with lymph node metastasis. Besides, the LOH results of FHIT, E-cadherin, and p53 genes were more frequently detected in papillary carcinomas showing metastasis or extrathyroidal tumor invasion, but without statistical significance. These results suggest that MSAs on various TSGs may contribute to the malignant transformation of follicular cells and malignant thyroid tumor progression, independently.
Our study has several limitations. First, we could not evaluate LOH in other thyroid cancers including medullary and anaplastic cancers. This may miss the difference in the clinicopathological features and biological behavior of various tumors arising within the thyroid gland. Secondly, only two microsatellite markers were used per gene, but it is considered that the LOH in the five TSGs are simply applicable markers with confidence, in comparing benign and malignant tumors clinically.
In this study, we demonstrated that LOHs were detected more frequently in thyroid carcinomas than in adenomas, in all five TSGs. Moreover, in papillary carcinoma, LOH in p16, E-cadherin, and p53 genes were significantly correlated with poorly differentiated grade. LOH results of FHIT, E-cadherin, and p53 genes were more frequently detected in papillary carcinoma showing lymph node metastasis or extrathyroidal tumor invasion. To conclude, the LOH in TSGs may independently contribute to the malignant transformation of follicular cells and malignant thyroid tumor progression, and that it could be used clinically as helpful markers in differentiating benign and malignant tumors. Also, use of the five LOH markers may help predict the clinicopathological features in papillary thyroid carcinoma patients.
Notes
No potential conflict of interest relevant to this article was reported.