Unidentified Bright Objects on Brain Magnetic Resonance Imaging Affect Vestibular Neuritis
Article information
Abstract
Objectives
The aim of this study was to investigate the differences in clinical manifestations of in two groups of vestibular neuritis (VN) patients with or without unidentified bright objects (UBOs).
Methods
A prospective, observational study with 46 patients diagnosed with VN between May 2013 and November 2013 was executed. A caloric test, a cervical vestibular-evoked myogenic potentials (cVEMPs) test, brain magnetic resonance imaging (MRI), spontaneous nystagmus test, head impulse test, and head-shaking nystagmus test were performed.
Results
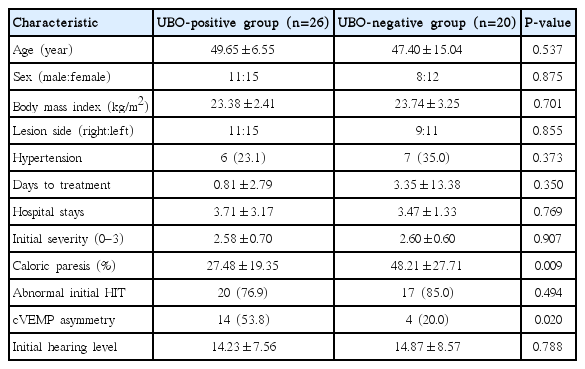
Of the patients, 56.5% (n=26) were classified as UBO-positive by MRI. These showed lower caloric weakness and more prominent cVEMP asymmetry compared with the UBO-negative group (P<0.05). Total VN (TVN) was the most common in the UBO-positive group (45.0%), followed by superior VN (SVN, 30.0%), and inferior VN (IVN, 25.0%). However, in the UBO-negative group, SVN (75.0%) was the most common, followed by TVN and IVN (P<0.05). The recovery rate was not influenced by UBOs (P>0.05).
Conclusion
UBOs on T2-weighted or fluid attenuated inversion recovery MRI may affect the patterns of the vestibular nerve in patients with VN.
INTRODUCTION
Vestibular neuritis (VN) patients complain of the acute onset of persistent rotatory vertigo with oscillopsia, gait and postural imbalance, tendency to fall, nausea and/or vomiting [1]. Although the causes of VN are not clear, suspected causes include the reactivation of latent herpes simplex virus type 1, an autoimmune abnormalities, or microvascular ischemia [2].
Magnetic resonance imaging (MRI) is usually performed in cases with a suspected central lesion. Although MRI alone is not useful for determining the cause of dizziness, MRI combined with other audio-vestibular tests is effective for evaluating potential retrocochlear lesions and brainstem lesions [34].
Nonspecific white matter hyperintensity signals on T2-weighted or fluid attenuated inversion recovery (FLAIR) MRI are often observed. These are referred to as unidentified bright objects (UBOs). UBOs are commonly found in the cerebellum, brain stem, thalamus, and basal ganglia [56].
Although the causes of UBOs are not fully understood, microangiopathic changes with reduced regional cerebral blood flow are believed to be associated with UBOs [78]. Elderly patients with diabetes, hypertension, and cerebrovascular disease are at high risk for UBOs [89].
Thus, VN and UBOs may share similarities in that microvascular changes are involved. However, we found that few study has been performed that classifies the characteristics of VN according to accompanying UBOs. Therefore, we hypothesized that VN patients may show differences in clinical manifestations, laboratory findings, bedside tests and prognosis according to the presence of UBOs as detected by MRI. Here, we describe a prospective, case-control study.
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board of Eulji University. Written informed consent was obtained from all patients.
Between May 2013 and November 2013, we recruited 46 patients who visited "dizzy clinics" at a university hospital with acute onset of dizziness and were diagnosed with VN and admitted for treatment. All patients underwent laboratory tests, bedside tests including spontaneous nystagmus (SPN), the head impulse test (HIT), head-shaking nystagmus (HSN), and brain MRI, which was obtained at the initial visit. A caloric test was also performed within three days of hospitalization.
The diagnosis of VN was obtained by fulfilling the criteria for inclusion: (1) a single acute onset of sustained vertigo, (2) unidirectional mixed horizontal-torsional SPN to the healthy side and following Alexander's law by infrared video goggle examination, (3) no additional neurologic signs or symptoms suggestive of other peripheral vestibular disorders or central lesions such as vertical divergence, perverted HSN, direction changing gaze-evoked nystagmus, or vertical nystagmus, and (4) exclusion of stroke, dementia, Parkinson disease, multiple sclerosis, diffuse axonal injuries, and cognitive deficit [21011].
Age, sex, presence of hypertension, the duration from onset to treatment, hospital stays, initial dizziness severity, and follow-up periods were documented. Hypertension was diagnosed with the patients' medical history and/or a systolic pressure of more than 140 mmHg or a diastolic pressure more than 100 mmHg during hospitalization periods.
For control of vertigo and nausea, oral dimenhydrinate 50 mg two times a day and intravenous metoclopramide 20 mg/day was administered for a maximum of five days. Vestibular rehabilitation therapy was performed and the patients were educated before discharge.
Assessment of vertigo severity
To assess the patients' vertigo severity, a modified version of a previously reported scale was used: 0, no static and dynamic symptoms; 1, mild vertigo with head movement; 2, severe vertigo with nausea; 3, severe vertigo with nausea and vomiting [11].
SPN, HSN, and HIT
For the measurement of SPN, the patient sat in the upright position with his/her gaze located in the center, and infrared goggles were used to assess SPN. The presence of SPN was considered to be positive regardless of intensity or direction [12]. For HSN, the patient's head was tilted forward at 30 degrees, and then shaken 20 times over 10 seconds at a speed of 2 Hz. An increased frequency of preexisting SPN was considered to be positive [12]. For HIT, the patient was asked to fixate his/her focus on the examiner's nose while rotating the patient's head 20 degrees to the right and left. Corrective saccades were considered to be positive [12]. These three tests were performed at both the initial visit and during every follow-up visit.
Caloric test
To evaluate horizontal canal function, a bithermal caloric test was performed and recorded by videonystagmography (CHARTR VNG, ICS Medical, Schaumburg, IL, USA) [1213]. Alternative irrigation using warm water (44℃) and cold water (30℃) was conducted for 30 seconds [1213]. Canal paresis (CP) was calculated using the Jongkees formula. More than 25% CP was considered to be pathologic.
Cervical vestibular evoked myogenic potentials
The same audiologist continuously conducted the cVEMPs test. The audiologist employed Navigator Pro software (Bio-logic Systems Co., Mundelein, IL, USA) and assessed VEMP responses by playing a 500-Hz tone burst at 95 dBnHL (decibel above normal adult hearing level) to test the ears through a headphone. The stimulation pattern used had a rise/fall time of 4 milliseconds and a plateau time of 2 milliseconds. The patient's head was tilted to the opposite side in the supine position to maximize activation of the sternocleidomastoid muscle. An active electrode was placed at the midpoint of both sternocleidomastoid muscles. A reference electrode was placed on the sternum. A ground electrode was placed on the central forehead. cVEMP amplitude asymmetry between both ears was calculated. cVEMP asymmetry exceeding 34% or no cVEMP response was considered to be significant asymmetry [14].
MRI protocol & detection of UBOs
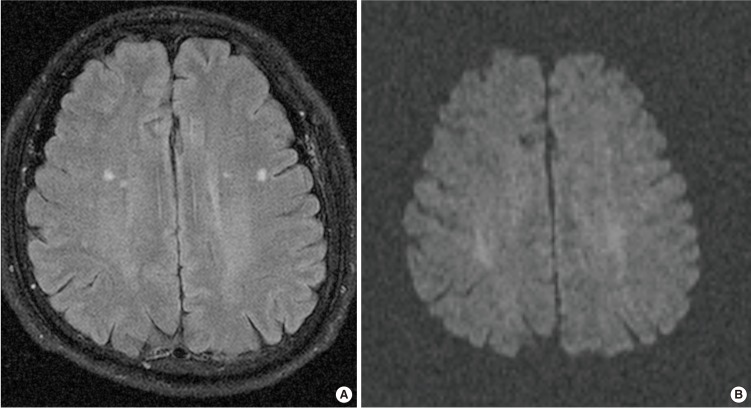
MRI was performed on either a 3T system (MAGNETOM Skyra, Siemens, Erlangen, Germany) or a 1.5T system (SONATA, Siemens) with a standard head coil. MRI sequences included an axial T1-weighted, axial T2-weighted, sagittal T1-weighted and T2-weighted, FLAIR images, diffusion weighted images (DWI), and with or without subsequent contrast enhancement spin-echo T1 W images. MRI parameters were as follows: 250-486/3.2-7.7 ms/70-75'/512-259 or 256×168 (TR/TE/FA/matrix) for T1-weighted images; 4,500-4,770/108-125 ms/132-150'/384×384 or 256×179 (TR/TE/FA/matrix) for T2 W images; 6,240-9,000/95-122 ms/150'/256×224 or 512×259 (TR/TE/FA/matrix) for FLAIR images; 4,500-8,200/98-99 ms/90'/128×127 or 192×154 (TR/TE/FA/matrix) at b=0 or 1,000 second/mm2 for DWI. The other parameters were same in both scanners: section thickness, 5 mm with a 1-mm gap and field of view of 175×200 mm. One neuro-radiologist with more than of 20 years of clinical experience reviewed the brain MRIs. The presence of UBOs was detected after differentiation of not only dilated perivascular spaces and old lacunes, which can be seen as bright spots on T2-weighted images by using a nulling fluid signal on FLAIR MRI, but also acute infarcts by DWI (Fig. 1).
Follow-up period & definition of complete recovery
After discharge from hospital, the patients were instructed to visit the dizzy clinic at 1 week, 2 weeks, 4 weeks, 6 weeks, 12 weeks, and 24 weeks from the discharge date for follow-up. Complete recovery was defined as when patients did not feel dizzy any more in their daily life and had a vertigo severity score of zero.
Statistical analysis
All of the statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA). Kolmogorov-Smirnov test and Shapiro-Wilk tests were performed to assess normality. Independent t-tests and Mann-Witney U-tests were used to assess between group differences. Chi-square analysis was performed to compare group differences between nominal variables. Kaplan-Meier analysis was used to compare survival curves. Forward conditional Cox proportional hazards models were used to calculate the hazard ratio of recovery and the 95% confidence interval (CI). P-values of less than 0.05 were considered statistically significant.
RESULTS
We enrolled 46 patients with VN, 19 males and 27 females with a mean age 49.06±10.31 years. The mean follow-up period was 27.41±37.45 days. The enrolled patients' characteristics, grouped by the presence of UBOs, are listed in Table 1. UBOs on brain MRI were detected in 26 patients (56.5%). With regard to location of UBOs, UBOs were the most commonly detected at fronto-parietal periventricular white matter (PVWM) and subcortical white matter (SCWM), followed by frontal PVWM, SCWM area, and fronto-parieto-temporal PVWM, SCWM area (Fig. 2). Two patients also had UBOs at the external capsule which contains corticocortical association fibers connecting the cerebral cortex to another cortical area.
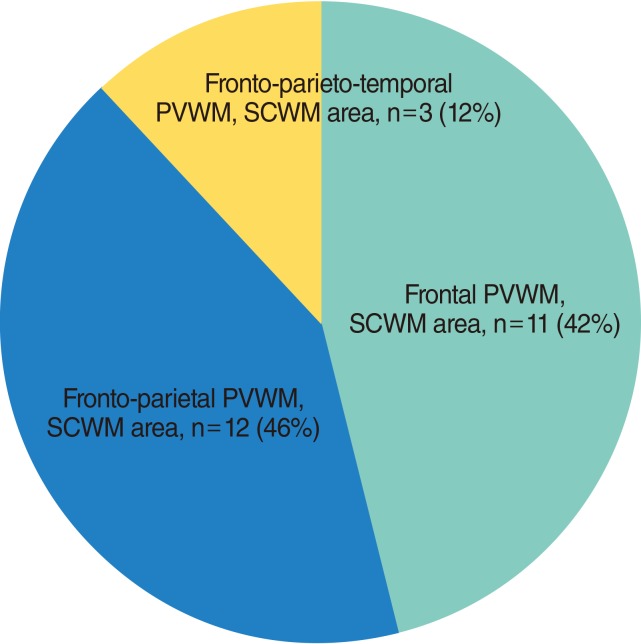
Distribution of unidentified bright objects in brain magnetic resonance imaging. PVWM, periventricular white matter; SCWM, subcortical white matter.
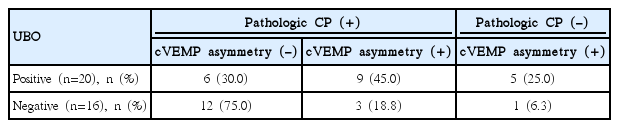
Aside from 10 patients who showed a normal caloric test and cVEMP symmetry, patients were classified into three groups according to these tests results (Table 2). Although 2 cells (33.3%) have an expected count less than 5, the test results were significantly different depending on the presence of UBOs (P=0.026, chi-square value=7.312). We performed a 2×2 chi-square analysis using the presence of UBOs and the cVEMPs results, but not the caloric test results. In the UBO-positive group, 14 of 20 patients (70.0%) showed significant cVEMP asymmetry, while only 4 of 16 (25.0%) in the UBO-negative group had cVEMP asymmetry (P=0.007, chi-square value=7.200).
The complete recovery rate of the UBO-negative group after 6 weeks was 75.1%, while the UBO-positive group was 69.0%. A log rank test revealed that this difference was not significant (P=0.839) (Fig. 3).

Kaplan-Meier plots of patients according to the presence of unidentified bright objects (UBOs) in brain magnetic resonance imaging (MRI).
During the follow-up period, SPN, HIT, HSN and a detailed assessment of vertigo severity was repeatedly performed. Patients with normal HIT showed a 1.972 times higher possibility of recovery (95% CI, 1.006 to 3.876). However, others tests were not significantly associated with recovery (P>0.05).
DISCUSSION
UBOs are considered an important pathologic condition, not a normal finding with ageing [8]. UBOs are more commonly found in patients with ischemic stroke or intracerebral hemorrhage than in healthy controls and are considered a strong predictor of functional outcome [151617]. These traits may be attributed to the assumption that UBOs imply collateral vessel damage or risk of new vascular events [1516]. In addition, the pathogenesis of UBOs is associated with small white matter infarcts in the vulnerable periventricular region [15].
Elderly patients with UBOs have increased risk of incidental falls, which may be caused by an interaction among UBOs, quadriceps muscle strength, and gait variability [18]. Prevention of additional white matter damage by control of cerebrovascular risk factors may lead to decreased numbers of incidental falls [19].
Age and hypertension are considered the leading risk factors associated with UBO progression [17]. However, in this study, the presence of hypertension was not significantly different in patients with and without UBOs (Table 1). For this reason, we assumed that the diagnosis of hypertension depended mainly on the statements of patients. In addition, the relatively small sample size and short hospital stays may not have been sufficient to identify hidden hypertension, which may have influenced this result.
Next, we found that patients with UBOs tend to have lower CP and prominent cVEMP asymmetry (Tables 1, 2). The calorictest is the gold standard to detect peripheral vestibular deficits and is useful to localize or quantify lesions [132021]. Commonly, patients with normal caloric test results are considered to have intact peripheral vestibular function [10]. However, the caloric test can only evaluate the low-frequency range of the vestibular-ocular reflex (up to 0.003 Hz) [22]. Moreover, inferior VN (IVN) sparing the horizontal canal may present with a normal caloric test finding [223] because the caloric test can evaluate only the regions innervated by the superior vestibular nerve or the horizontal semicircular canal [24]. Other factors that can influence the caloric test results include the morphology of the ear, pneumatization of mastoid cells, and the patient's alertness [24].
Affected branches of the vestibular nerve can be estimated by cVEMPs and ocular VEMPs (oVEMPs) that evaluate saccular and utricular function independently [3]. Superior VN (SVN) patients show typical CP, abnormal oVEMPs responses, and normal cVEMPs response [23]. On the other hand, IVN patients show a normal caloric test, oVEMPs response and intact horizontal HIT as well as an abnormal cVEMP response. Total VN (TVN) patients may show abnormalities on all test results mentioned above.
When applying these concepts to the results of study, pathologic CP (+), cVEMP asymmetry (-), pathologic CP (-), cVEMP asymmetry (+), pathologic CP (+), cVEMP asymmetry (+) in Table 2 correspond to SVN, IVN, and TVN.
Aside from 10 patients who showed normal CP and cVEMP symmetry, the UBO-negative group consisted of mostly SVN (75.0%), followed by TVN (18.8%) and IVN (6.3%). On the contrary, TVN was most common in the UBO-positive group, followed by SVN (30.0%) and IVN (25.0%) (P=0.026) (Table 2). These results imply that the presence of UBOs may accompany a difference in the distribution of the affected branch although the direct mechanism remains unclear. Our finding that 70% of the UBO-positive group had cVEMP asymmetry suggests that UBOs may be associated with the inferior vestibular nerve.
Generally, the superior vestibular nerve is considered to be more susceptible to entrapment and ischemia because it runs through a longer, more narrow bony channel with more interspersed bony spicules than the inferior or singular nerve [25]. In addition, Goebel et al. [26] reported that the mean ratio of arteriole (A) to arteriolar canal (AC) width of the singular nerve (A:AC ratio=0.45) was lower than of the superior vestibular nerve (A:AC ratio=0.54), and this suggested that the arteriole following the superior vestibular nerve runs within a narrower bony channel.
From an anatomic perspective, the labyrinthine artery divides the anterior vestibular artery and the common cochlear artery [27]. The former provides a blood supply to most of the regions innervated by the superior vestibular nerve. The latter divides the proper cochlear artery and the vestibulo-cochlear artery. The posterior ampulla and most of the saccule that are innervated by the inferior vestibular nerve have a blood supply from the posterior vestibular artery, which is the terminal branch of the vestibulo-cochlear artery.
Given the findings, we assumed that the terminal branch such as the posterior vestibular artery (feeding the inferior vestibular nerve) in patients with UBOs may be more selectively vulnerable to transient vascular ischemia and small vessel disease. This could explain the high prevalence of cVEMP asymmetry and the lower frequency of abnormal CPs in UBO patients. To confirm this hypothesis, additional anatomical and radiologic studies would be necessary.
The study limitations are as follows. First, we did not repeat cVEMPs test for follow-up and other tests such as oVEMPs, subjective visual verticals, and/or vertical HIT were not performed. Identification of the affected branches by the caloric and cVEMPs tests at initial evaluation alone may not be sufficient. Moreover, 10 patients showed normal CP and cVEMP symmetry and were excluded (Table 2). Our sample size is not small considering that a total of 46 patients were enrolled in this study. Therefore, more accurate localization may be possible only if we were to perform more diverse newly developed vestibular tests [2328].
In addition, we did not perform quantitative analysis of UBOs and depended entirely on the radiologist's reading of whether UBOs were present. Further research should include additional data including the size, and extent of UBOs.
In conclusion, UBOs may influence the patterns of the affected vestibular nerve in patients with VN. The inferior vestibular nerve seems to be more commonly affected in patients with UBOs. Our results may be explained by the characteristics of UBOs, as one of the small vessel diseases: the posterior vestibular artery (which supplies blood to the inferior vestibular nerve), which is a terminal branch, may be more influenced by UBOs than the superior vestibular artery (which supplies blood to the superior vestibular nerve).
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.