Tumor Regression Patterns Based on Follow-up Duration in Patients With Head and Neck Squamous Cell Carcinoma Treated With Radiotherapy or Chemoradiotherapy
Article information
Abstract
Objectives
We describe patterns of tumor regression based on follow-up duration after radiotherapy (RT) or chemo-RT in patients with head and neck squamous cell carcinoma.
Methods
Thirty-one patients with head and neck squamous cell carcinoma were included in this study and received definitive RT or chemo-RT. The pattern of primary tumor regression after treatment was evaluated every 1 to 2 months. Predictive factors for the length of time to full regression were also analyzed.
Results
Among all patients, 27 patients showed regression of the primary tumor, 24 patients showed >50% regression, and 15 patients showed total regression. The primary tumor gradually regressed during the course of follow-up. The median time to full regression was 5.2 months (range, 1.3 to 17.9 months). In the 24 patients who showed >50% regression, the rate of >50% regression increased over time as follows: 25.0% at 1 month, 62.5% at 2 months, 75.0% at 3 months, 91.7% at 4 months, and 95.8% at 5 months. Higher total RT dose and shorter RT duration were associated with longer time to full regression.
Conclusion
A substantial number of patients showed continuous regression of the primary tumor for more than 2 months after treatment. The timing for evaluation of tumor regression must be greater than 2 months from the completion of RT or chemo-RT in patients with head and neck squamous cell carcinoma.
INTRODUCTION
The aim of follow-up for patients with head and neck cancer treated with radiotherapy (RT) or chemo-RT is early detection of residual or recurrent tumors. Early diagnosis of residual or recurrent tumors is important in order to increase the efficacy of salvage treatment [12]. Various surveillance schemes have been proposed for follow-up of patients with head and neck cancer treated with RT or chemo-RT [3456]. However, there is no definite consensus on what surveillance scheme is most effective.
In order to effectively conduct surveillance in patients with head and neck cancer treated with RT or chemo-RT, it is important to understand patterns of tumor regression based on follow-up duration after treatment. However, until now, no study has reported tumor regression patterns according to follow-up duration after RT or chemo-RT in patients with head and neck cancer. In this study, we attempt to describe the patterns of primary tumor regression according to follow-up duration after RT or chemo-RT in patients with head and neck squamous cell carcinoma.
MATERIALS AND METHODS
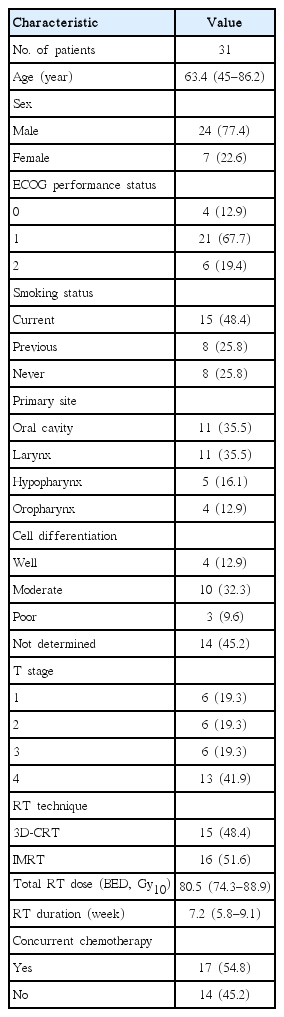
Criteria for patient eligibility included histologically confirmed head and neck squamous cell carcinoma, receipt of definitive RT or chemo-RT, good general condition with Eastern Cooperative Oncology Group performance status ≤2, no previous history of head and neck area irradiation, no distant metastases, follow-up duration ≥12 months, and available follow-up data. The patients who received RT or chemo-RT after surgical resection and patients who received induction chemotherapy were excluded. The patients who received palliative RT were also excluded. From January 2007 to July 2013, 133 patients with head and neck cancer received RT or chemo-RT at our institution. Of these patients, 31 met the eligibility criteria and were included in this study.
Pretreatment evaluation consisted of a complete history and physical examination, pan-endoscopy, complete blood counts, liver and renal function tests, dental evaluations, computed tomography (CT) scans and/or magnetic resonance imaging (MRI) of the head and neck region, and positron emission tomography. Bone scans and CT scans of the abdomen and/or chest were performed only when clinically indicated. The cancer stage of each patient was assigned based on the American Joint Committee on Cancer staging system (7th edition). Histologic grade was described according to the World Health Organization classification. For all patients, we retrospectively reviewed hospital records, laboratory results, and imaging studies. The Institutional Review Board of the Kyung Hee Univesity Hospital approved this study (IRB No. KMC 1432-03), and all research was carried out in compliance with the Helsinki Declaration.
All patients received CT-planned RT with either three-dimensional conformal RT (3D-CRT) or intensity-modulated RT (IMRT) technique. The choice between 3D-CRT and IMRT was determined by the physician who also taking the patient's interests into account. The gross tumor volume (GTV) was defined as the gross extent of the primary tumor and grossly involved cervical lymph nodes. High-risk clinical target volume (CTV) was defined as the GTV plus a 1- to 1.5-cm margin to account for subclinical tumor spread. Low-risk CTV was defined as the total volume of prophylactically treated neck lymph nodes. The planning target volume (PTV) was created by adding an additional 5-mm margin to the CTV to account for setup errors. The prescription dose was determined by the physician and was based on tumor stage, the patient's general condition, and the probability of RT-induced toxicity. High-risk PTV was treated with a daily dose of 1.8-2.2 Gy and a total dose of 61.6-73.5 Gy. The most commonly prescribed dose fractionation schedule was a total dose of 66 Gy with a daily dose of 2.2 Gy. Among the 31 patients, 10 patients (32.3%) were treated with this dose fractionation schedule. Low-risk PTV was treated with a daily dose 1.65-2.1 Gy and a total dose of 45-56.1 Gy. 3D-CRT was carried out on a Clinac iX (Varian Medical System Inc., Palo Alto, CA, USA). IMRT was carried out on a TomoTherapy (TomoTherapy Inc., Madison, WI, USA) with simultaneous integrated boost technique. Treatment plans were evaluated using a dose-volume histogram and visually inspecting isodose curves. In general, we considered plans to be acceptable if the PTV was covered by 95% isodose curves, inhomogeneity of the PTV ranged from 95% to 107%, and doses to critical normal structures were limited in their tolerances. Systemic chemotherapy was not given routinely, but was individualized based on tumor stage, physician's preference, and patient's performance status and compliance. The most common concurrent chemotherapy regimen was cisplatin (100 mg/m2) for three cycles during RT.
The pattern of primary tumor regression was evaluated by CT and/or MRI every 1 to 2 months. All images were interpreted by a radiologist with more than 10 years of experience reviewing CT and MRI of the head and neck regions. Total regression was defined as the disappearance of any intratumoral arterial enhancement, and >50% regression was defined as at least a 50% decrease in the sum of the diameters of viable primary tumor. Full regression was defined as an enhanced primary tumor lesion did not become smaller any more. All patients were evaluated until the primary tumor reached total or full regression. Time to total regression, >50% regression, and full regression was calculated from the date of RT completion to the date of imaging study corresponding to the final determination of each regression status. In-field locoregional recurrence was defined as an increase in the size of target lesions or the appearance of new lesions within the PTV. Out-field locoregional recurrence was defined as the appearance of new lesions outside of the PTV in the head and neck region. Distant metastasis was defined as evidence of tumor in any other area. Actuarial rates were estimated using the Kaplan-Meier method, and comparisons between groups were performed using log-rank tests. Factors that influenced the length of time to full regression were analyzed. Parameters evaluated as potential predictive factors for time to full regression were sex, age, smoking status, T stage, RT technique, total RT dose, daily RT dose, RT duration, RT interruption, and concurrent chemotherapy. For multivariate analysis, the Cox proportional regression hazard model was used. For all analyses, a P-value <0.05 was considered statistically significant. All analyses were performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
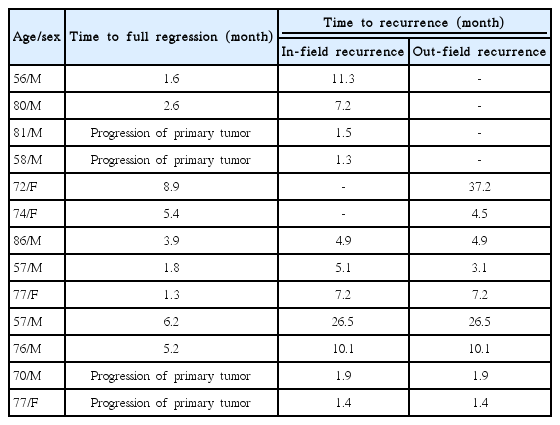
Patient and tumor characteristics are summarized in Table 1. Five patients (16.1%) were stage I, 3 (9.7%) were stage II, 4 (12.9%) were stage III, 13 (41.9%) were stage IVA, and 6 (19.4%) were stage IVB. Eight patients (25.8%) experienced temporary RT interruption because of treatment toxicity, and the median interruption duration was 4 days (range, 3 to 24 days). During the follow-up period, 24 patients (77.4%) were still alive and 7 patients died due to disease progression. The median follow-up period was 24.1 months (range, 8.2 to 86 months) for all 31 patients and 27.5 months (range, 12 to 86.0 months) for the surviving patients. Distant metastases developed in 4 patients (12.9%). The metastatic sites were lung in 2 patients, bone in 1 patient, and lung and liver in 1 patient. Loco-regional recurrences developed in 13 patients (41.9%).
The status of primary tumor regression after treatment is summarized in Fig. 1. Among all patients, 4 (12.9%) showed an increase in the total diameter of the viable primary tumors on the first follow-up imaging study, while 27 (87.1%) showed regression of the primary tumor. The primary tumor gradually regressed throughout the course of follow-up. The median time to full regression was 5.2 months (range, 1.3 to 17.9 months) (Fig. 2). Among the 27 patients who showed regression of the primary tumor, 24 patients showed >50% regression of primary tumor, and 15 patients eventually experienced total regression of primary tumor during the follow-up period. The median time to >50% regression of primary tumor was 1.9 months (range, 0.7 to 5.2 months). In the 24 patients who showed >50% regression, the rates of >50% regression increased over time as follows: 25.0% at 1 month, 62.5% at 2 months, 75.0% at 3 months, 91.7% at 4 months, and 95.8% at 5 months. The latest >50% regression occurred at 5.2 months (Fig. 3). The rates of total regression also increased over time, and the median time to total regression of primary tumor was 4.1 months (range, 1.3 to 15.9 months).
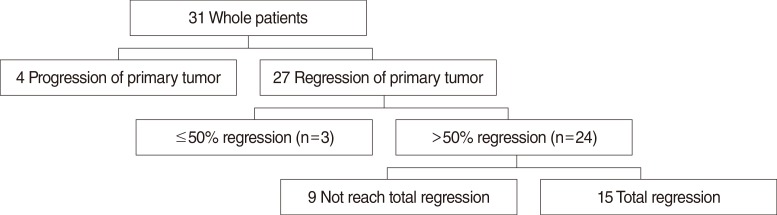
Schematic summary of primary tumor regression after radiotherapy or chemoradiotherapy in patients with head and neck squamous cell carcinoma. Among all patients, 27 patients showed regression of primary tumor, 24 patients showed >50% regression, and 15 patients showed total regression.

Patterns of primary tumor regression in 27 patients with head and neck squamous cell carcinoma who showed regression of primary tumor after radiotherapy or chemoradiotherapy. The median time to full regression was 5.2 months (range, 1.3 to 17.9 months).
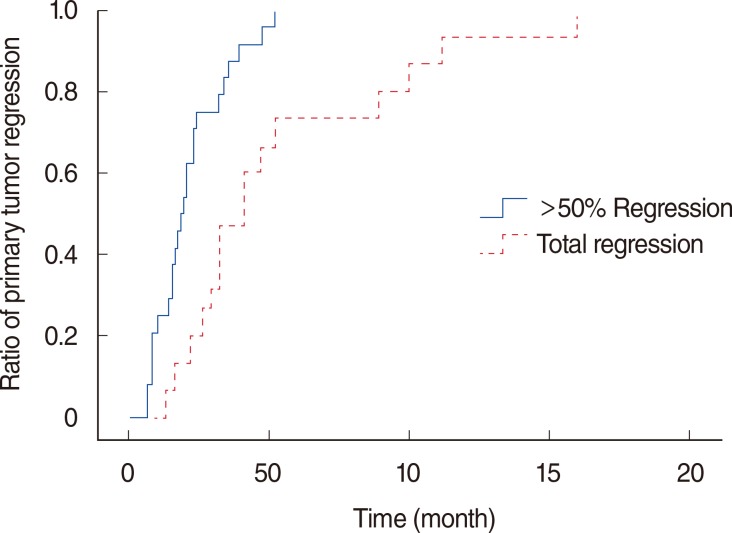
Development patterns of >50% and total primary tumor regression based on follow-up duration after radiotherapy or chemoradiotherapy in 24 patients who showed >50% regression and 15 patients who showed total regression. The rates of >50% and total regression increased over time. The median time to >50% and total regression were 1.9 months (range, 0.7 to 5.2 months) and 4.1 months (range, 1.3 to 15.9 months), respectively.
Among all patients, 13 patients (41.9%) experienced loco-regional recurrences. Four patients experienced in-field recurrences, 2 patients experienced out-field recurrences, and 7 patients experienced both in- and out-field loco-regional recurrences. The development patterns of loco-regional recurrence were summarized in Table 2. Except for four patients who showed a progression of primary tumor on the first follow-up imaging, all patients experienced in-field recurrences after full regression of primary tumor, and only one patient experienced out-field recurrence before full regression of primary tumor.
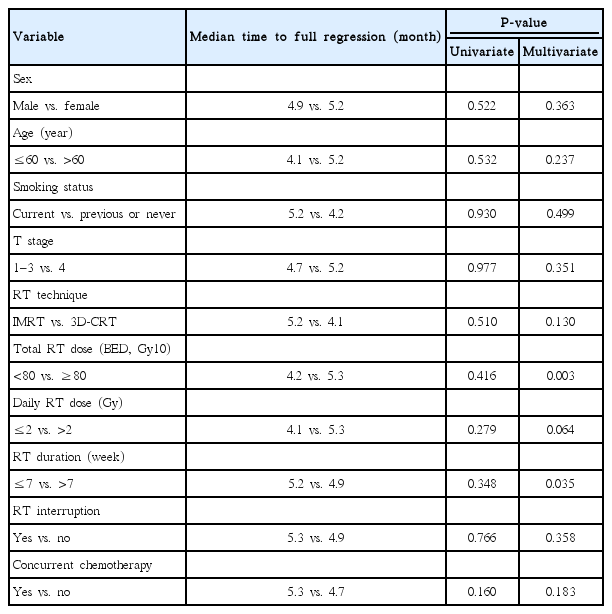
We analyzed the factors that influenced the length of time to full regression. In univariate analysis, there was no factor that significantly associated with the length of time to full regression. However, in multivariate analysis, total RT dose (hazard ratio, 0.083; 95% confidence interval, 0.016 to 0.428, P=0.003) and RT duration (hazard ratio, 4.844; 95% confidence interval, 1.114 to 21.067, P=0.035) were significantly associated with time to full regression (Table 3). Higher total RT dose and shorter RT duration were associated with a longer time to full regression.
DISCUSSION
The diagnosis of recurrent or residual head and neck cancer previously treated by RT or chemo-RT remains a challenging task. Because postradiation changes in the primary site may obscure tumor measurements, posttreatment evaluation can be complicated and difficult to interpret [78]. Therefore, many physicians perform endoscopic evaluation with biopsy under anesthesia to allow for accurate assessment of suspected recurrent or residual head and neck cancer. However, multiple biopsies performed to rule out the presence of a tumor may cause trauma to heavily radiated tissue and exacerbate postradiation changes [1]. As a result, the risk of complication and the chance of missing recurrent tumor may increase. In this study, we examined the patterns of primary tumor regression based on follow-up duration and calculated the time from completion of RT to full regression of primary tumors in patients with head and neck squamous cell carcinoma. Although time to full regression varied by individual, it took longer than 5 months for primary tumors reach full regression in nearly half of the patient population (Fig. 2). Among the 27 patients who showed regression of primary tumors, only 7 patients (25.9%) showed full regression within 3 months after treatment. Because RT must be allowed to have full effect before evaluation of treatment outcome, frequent biopsies should be carefully implemented earlier in the course of follow-up after RT or chemo-RT in patients with head and neck squamous cell carcinoma to avoid over-treatment and unnecessary trauma in heavily radiated tissue.
Imaging plays an important role in posttreatment evaluation of patients with head and neck cancer, and among a variety of imaging modalities, CT and MRI are the most popular for follow-up because of rapid image acquisition and superior contrast resolution [910]. Many researchers argued that total radiologic regression is indicative of cure, whereas <50% reduction of the primary tumor is indicative of treatment failure [1101112]. However, until now, there have been no reports addressing patterns of primary tumor regression based on follow-up duration, so it remains unknown when the degree of primary tumor regression should be evaluated after RT or chemo-RT in patients with head and neck cancer. The timing of CT and MRI for evaluation of the degree of primary tumor regression after treatment is an important issue because it correlates with diagnostic accuracy [1314]. Although many researchers have suggested that CT and/or MRI should be performed 1-2 months after completion of treatment to evaluate the degree of primary tumor regression [1101415], we think that an interval longer than 2 months may be needed to precisely evaluate the primary tumor regression. In our study, of the 24 patients who showed >50% primary tumor regression during the follow-up period, 9 patients (37.5%) did not show >50% regression before 2 months of RT completion. Despite these 9 patients showed <50% regression of the primary tumor at the time of 2 months after completion of treatment, 5 patients eventually showed total regression of the primary tumor thereafter, and only 1 patient experienced loco-regional recurrence during the follow-up period. Also, among the 15 patients who showed total primary tumor regression during follow-up period, only 2 patients showed total regression at 2 months. The other 13 patients (86.7%) showed total regression of the primary tumor after 2 months of RT completion, and none of these patients experienced loco-regional recurrence during the follow-up period. Therefore, because the primary tumor must be allowed to fully regress before evaluation of treatment outcome, the timing of evaluation of tumor regression must be longer than 2 months from the completion of RT in patients with head and neck squamous cell carcinoma. Further studies with a larger sample size should be conducted to determine the optimal timing of CT and MRI for evaluation of the degree of primary tumor regression after RT or chemo-RT in patients with head and neck squamous cell carcinoma. We hope this study will serve as a stepping stone for future studies.
This is the first study that analyzed predictive factors for the length of time to full regression of primary tumor after RT or chemo-RT in patients with head and neck cancer. According to our study, higher total RT dose (80 Gy10 or more) and shorter RT duration (7 weeks or less) were significantly associated with longer time to full regression in multivariate analysis. Therefore, to precisely evaluate primary tumor regression in these patient groups, longer follow-up duration is required. The reasons for longer time to full regression after RT or chemo-RT in these patient groups have not yet been investigated. To confirm the results of our study, further studies are warranted.
There were some limitations in this study. First, this study was retrospective and may have inherent biases. For example, evaluations of primary tumor regression were conducted at the physician's discretion rather than based on an established protocol, so the time of imaging study acquisition varied among the enrolled patients. However, to more effectively evaluate the regression pattern of the primary tumor, we frequently conducted CT and/or MRI (every 1 to 2 months) in all enrolled patients. Second, because of incomplete patient medical records, we could not analyze tumor cell differentiation as a potential predictive factor for the length of time to full regression of the primary tumor. Third, the sample size was small, so we may not have detected minor differences in statistical analysis. However, as the first study to report on the pattern of tumor regression based on follow-up duration in head and neck cancer, we believe that our study provides some evidence for optimal evaluation time of primary tumor regression in patients with head and neck squamous cell carcinoma treated with RT or chemo-RT.
In conclusion, primary tumor gradually regressed after RT or chemo-RT in patients with head and neck squamous cell carcinoma. A substantial number of patients showed continuous regression of primary tumors for more than 2 months after treatment. The timing for evaluation of tumor regression must be greater than 2 months from the completion of RT or chemo-RT in patients with head and neck squamous cell carcinoma.
Notes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.