Use of 18F-Fluorodeoxyglucose Positron Emission Tomography in Patients with Rare Head and Neck Cancers
Article information
Abstract
Objectives
The clinical utility of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been demonstrated in major head and neck cancers (HNCs) but is unclear in rare HNCs. We therefore evaluated FDG PET in the management of patients with rare HNCs.
Methods
FDG PET and CT/MRI scanning were performed at the initial staging and/or the follow-up in 24 patients with rare HNCs, 10 with melanoma, 9 with sarcoma, 3 with olfactory neuroblastomas, and 2 with basal cell carcinoma. The diagnostic accuracy of CT and FDG PET for detecting primary tumors and metastases were compared with a histopathologic reference. The association between the PET results and the clinicopathologic parameters predicting tumor invasion, histologic grade and disease-free survival (DFS), was assessed.
Results
The overall accuracies of FDG PET and CT/MRI were 92% and 79%, respectively, for detecting primary tumors and 91% and 74%, respectively, for nodal metastases, but the differences were not significant due to the small number of patients. The sensitivity and specificity of FDG PET for detecting distant metastases and second primary tumors were 100% and 87%, respectively. Follow-up FDG PET correctly diagnosed locoregional recurrence in all 12 patients, as shown by biopsy, and distant metastases in 6 patients. However, thickness of melanoma, histologic grade of sarcoma, and DFS were not associated with tumor FDG uptake.
Conclusion
FDG PET may be useful for staging, posttreatment monitoring, and detection of distant metastases and second primary tumors in patients with rare HNCs.
INTRODUCTION
Head and neck cancers (HNC) are the sixth most common type of human cancer worldwide. Squamous cell carcinomas form the major type of the extracranial HNCs, followed by lymphomas and differentiated thyroid cancers (1). Due to the higher glycolytic rates of many neoplasms relative to normal tissue, the glucose analog 18F-fluorodeoxyglucose (FDG) can be used to detect tumors, and positron emission tomography (PET) using FDG has been utilized in patients with HNCs for initial staging, management of recurrent cancers, and therapeutic monitoring (2-5). Since head and neck tumors generally have high uptake of FDG (6), FDG PET can detect HNCs that might not be revealed by conventional imaging methods, including computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography (7-9). Although studies of salivary cancers, melanomas, basal cell carcinomas, and sarcomas arising in the head and neck region have suggested that FDG PET may be clinically useful in the staging, histologic grading, and post-treatment monitoring of patients with these tumors (10-13), the role of FDG PET in rare HNCs is still unclear, due to the rarity of these cancers. We therefore evaluated the role of FDG PET for staging and monitoring patients with rare HNCs, including melanomas, basal cell carcinomas, sarcomas, and olfactory neuroblastomas (esthesioneuroblastomas).
MATERIALS AND METHODS
Patient population and treatment
Patients presenting with rare HNCs between March 2000 and December 2005 were enrolled in this study. All patients with previously untreated or recurrent rare HNCs were assessed by biopsy, CT and whole body FDG PET before treatment for previously untreated or recurrent rare HNCs. Patients with squamous cell carcinoma, lymphoma, thyroid cancer, salivary gland cancer, nasopharyngeal carcinoma, metastatic lesion with primary tumor outside the head and neck, and metastasis from unknown primary squamous cell carcinoma or adenocarcinoma were excluded, as were patients not assessed by FDG PET prior to treatment or during follow-up and patients with inadequate follow-up information. For patients with more than one FDG PET scan, the pretreatment or the most relevant follow-up scan was included. Final diagnosis was based on pathologic confirmation of the tumor and adequate follow-up. This study was reviewed and approved by the Institute's Ethics Review Committee.
Twenty-four patients with skin cancer (n=12), sarcoma (n=9), or olfactory neuroblastoma (n=3) in the head and neck area were included in the final analysis (Table 1). The patient cohort consisted of 9 men and 15 women, of mean age 51 yr (range, 11-81 yr). FDG PET scanning was performed in 20 patients with previously untreated tumors during initial staging and in 4 with primary or cervical nodal recurrence at follow-up (4-18 months) after initial treatment. Staging of olfactory neuroblastomas and other HNCs were as described (Table 1) (14, 15).
Nineteen patients underwent resection of the primary tumor, with or without neck dissection. Four patients with cervical nodal recurrence of melanoma underwent only neck dissection. One patient with olfactory neuroblastoma refused definitive surgery and so received chemoradiotherapy. Supraomohyoid neck dissection (levels I-III) was performed in 11 patients suspected of having high-grade or locally invasive tumors and clinically negative nodal involvement. Modified or radical neck dissection (levels I-V) was performed in 11 patients suspected of having nodal involvement or extracapsular nodal spread. Bilateral neck dissection was performed in one patient suspected of having bilateral disease. Retropharyngeal nodes were dissected in 3 patients suspected of having metastases to the nodes and parotidectomy was performed at the tumor-affected sides of 4 patients. Seventeen of 24 patients (71%) received postoperative locoregional radiotherapy (mean dose, 63.4 Gy; range, 34.2-70.2 Gy), using single daily fractions, and 10 patients received chemotherapy.
Imaging
All patients underwent PET with an ECAT HR+ scanner (Siemens/CTI, Knoxville, TN, USA), with an in-plane spatial resolution of 4.5 mm and an axial field view of 15.5 cm. Patients fasted for at least 6 hr prior to FDG PET scanning, and their blood glucose concentrations measured; patients with diabetes mellitus had to have blood glucose levels below 150 mg/dL prior to the scan. All patients were rested for at least one hour before FDG PET scan. Beginning approximately 60 min after intravenous injection of about 555 MBq FDG, whole-body imaging was performed with the patient in the supine position. Data were reconstructed into coronal, sagittal, and transverse sections and a three-dimensional rotating projection. The standardized uptake value (SUV) was calculated using the attenuation-corrected images, the amount of injected FDG, the lean body weight of each patient, and the cross-calibration factors between FDG PET and the dose calibrator.
All patients also underwent CT of the head and neck, using a LightSpeed QX/i scanner (GE Medical Systems, Milwaukee, WI, USA) or a Somatom Sensation 16 (Siemens Medical Solutions, Forchheim, Germany), with a slice thickness of 3-5 mm. Patients were placed in the supine position, and CT was performed with contrast-enhanced axial images parallel to the occlusal line from the skull base to the upper chest. In selected patients, direct coronal or coronal reconstruction images were also obtained. MRI was performed in 8 patients with a 1.5-T unit (Siemens Medical Solutions) using the spin-echo technique and a slice thickness of 5 mm. Unenhanced T1-weighted images were acquired in the coronal and axial planes and T2-weighted fat-suppressed fast-spin-echo images were also obtained. After injection of 0.1 mmol gadolinium/kg body weight, T1-weighted fat-suppressed axial, coronal, and sagittal images were sequentially obtained.
Image interpretation and histological examination
All FDG PET images were re-reviewed by an experienced nuclear medicine staff member. The degree of suspicion of malignant involvement was based on qualitative visual interpretation of the images and the determination of maximal SUV, a semiquantitative measure of relative FDG uptake within the regions of interest. FDG PET images were reviewed to calculate the SUV, and the slice containing the tumor was selected. The maximum SUV of primary tumors was used as a reference for correlation with survival.
CT/MRI results were interpreted by experienced radiologists. Nodes were considered metastatic if central necrosis or inhomogeneous enhancement was present, if their longest axial diameter was over 1.5 cm in the jugulodigastic regions or over 1 cm in other cervical regions, or if there was a cluster of 3 or more lymph nodes of borderline size. The FDG PET and CT/MRI readers were blinded to each other's results, as well as to pathology results.
To correlate CT/MRI and FDG PET results, the primary tumor sites and the sides and levels of the involved neck or retropharyngeal nodes were recorded (16). Primary tumors and lymph nodes were dissected from the specimens and assessed histopathologically. Local invasiveness, the histological grade of the primary tumors, the total number of lymph nodes, and the presence or absence of tumor within these nodes and at the primary sites were recorded by an experienced pathologist.
Statistical analysis
The sensitivity, specificity, and predictive values of each imaging method for detecting primary tumors and metastatic nodes were calculated and compared with histological results. A two-tailed P value was used to assessed according to the FDG PET with CT/MRI. The χ2 test, or Mann-Whitney or Kruskal-Wallis test was used for categorical data or mean analyses. Correlation between maximal SUV and other significant variables (tumor size and thickness, histologic grade, stage) was analyzed using Spearman correlation coefficients (r).
Actuarial disease-free survival (DFS) and overall survival rates were calculated by the Kaplan-Meier method. Persistent or recurrent tumor was documented by CT/MRI or FDG PET scanning. The time interval for local control and survival endpoints was calculated from the first day of initial treatment until the date of an event or of the last follow-up. The log-rank test was used to assess the correlation of these endpoints with SUV and the clinicopathologic variables. A P value less than 0.05 was considered statistically significant.
RESULTS
Two patients had melanomas in cervical nodes but no proven primary sites. The most common sites of these rare HNCs were the nasal cavity and neck (Table 1). Three patients had stage I disease, five stage II, 12 stage III, and one stage IV; a patient with olfactory neuroblastoma was in Kadish stage B and two were in stage C disease. Ten patients (42%) had metastases to the cervical or retropharyngeal lymph nodes. Primary tumors were completely removed in 20 of the 23 patients who underwent surgery; their mean±SD size in the largest histopathologic dimension was 4.5±2.5 cm. Three patients had microscopic residual tumors at the surgical margins. The mean±SD thickness of primary melanoma was 3.6±2.7 mm; of the patients with sarcoma, 4 each had low and high grade tumors.
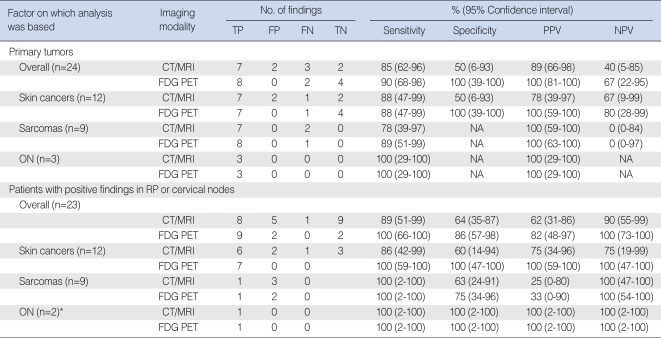
The overall sensitivity of FDG PET and CT/MRI for detecting primary tumors was 18/20 (90%) and 17/20 (85%), respectively, and their specificity was 4/4 (100%) and 2/4 (50%), respectively (Table 2). The positive and negative predictive values of FDG PET were 11% and 27% higher, respectively, than those of CT/MRI. None of these differences, however, was statistically significant using the McNemar test (P>0.05). CT/MRI was unable to detect definitive primary lesions in 3 patients with melanoma or sarcoma; of these, 2 were correctly detected by FDG PET (Fig. 1). CT/MRI also falsely diagnosed primary lesion sites of the 2 patients with cervical nodal melanomas with unknown primary site: both were negative on FDG PET. The latter method yielded no false-positives, but 2 false-negatives, one patient with melanoma and the other with an unclassified round cell sarcoma, with the largest tumor diameters less than 2.5 cm (Table 2). One of these primary lesions was detected by CT/MRI.
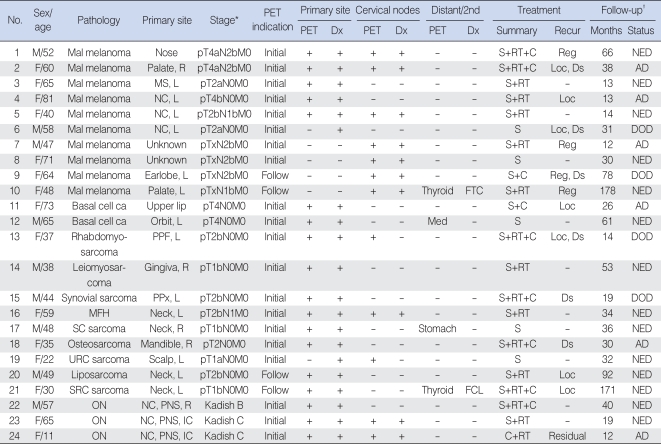
Results of CT/MRI and PET for detecting primary tumors and metastatic nodal diseases in patients with rare head and neck cancers
Detection of primary tumor and nodal metastasis by FDG PET. (A-C) Whole body FDG PET showing focal FDG uptake in the left anterior nasal cavity (arrowheads) and upper neck (arrows) of a 40-yr-old melanoma patient (case no. 5). (D, E) Axial CT scans showing the ab-sence of significant lesions in the nasal cavity and upper neck. Both lesions were confirmed by surgical pathology.
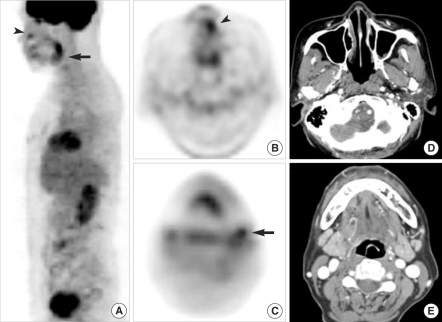
Ten of 23 patients (42%) had cervical (n=8) or retropharyngeal (n=2) nodal metastases: 9 were diagnosed by node dissection and 1 by fine-needle aspiration biopsy (case no. 24). When we analyzed the correlation of CT/MRI, FDG PET, and histology in the 23 patients who underwent surgery for nodal diseases, we found that the overall sensitivity, specificity, and positive and negative predictive values of FDG PET for detecting nodal metastases were 10-22% higher than those of CT, but the differences were not significant by the McNemar test (P>0.05) because of the small number of patients (Table 2). FDG PET accurately detected the presence or absence of nodal metastases in 21 of 23 patients (91%), whereas CT was accurate in 17 (74%). FDG PET falsely interpreted neck disease in 2 of 23 patients (7%); the two false-positives were due to reactive or inflammatory nodes and proximity to the primary lesion of a sarcoma patient (Fig. 2).

False results of both FDG PET and MRI. (A, B) Whole body FDG PET showing focal FDG uptake in the left posterior neck (black arrows) and no other sites of a 22-yr-old sarcoma patient (case no. 19). (C) Gadolinium-enhanced axial T1-weighted MR image showing a strongly enhancing lymph node (white arrow). Surgical pathology revealed a 1-cm-sized, round cell sarcoma in the skin and superficial subcutaneous tissue on the left scalp but no cervical nodal metastases. The positive node on PET and MRI was a reactive lymph node.
FDG PET scans suggested distant metastases and second primary cancers in 1 and 3 patients, respectively; of these, only one had a true second primary malignancy in the thyroid gland (case no. 10). The PET-positive findings were confirmed by further imaging work-ups and biopsy, and PET-negative findings by follow-up imaging. The overall sensitivity, specificity, and positive and negative predictive values of FDG PET for detecting distant metastases or second primary tumors were 100%, 87%, 25%, and 100%, respectively.
The maximum SUV was 5.1±3.0 (range, 1.4 to 12.0) in primary tumors and 6.1±3.4 in cervical diseases (range, 1.9-12.0). Mean tumor or nodal SUV did not correlate with age, sex, nodal positivity, or tumor pathology (P>0.05). The SUV of primary tumors did not statistically correlate with the size or stage of tumor, melanoma thickness, or the grade of sarcoma (P>0.05).
At last follow-up, 14 patients were alive without disease and 6 were alive with disease. The mean follow-up for surviving patients was 48.5±47.8 months (range, 12 to 178 months). Twelve patients presented with persistent or recurrent locoregional diseases on FNAB, CT/MRI and FDG PET and 6 presented with distant metastases; of these 4 died of diseases. At 4 years, actuarial locoregional control was 59%, DFS was 56%, and overall survival was 83%. Univariate analysis for DFS showed that age, sex, local invasion, thickness of melanoma, pathology grouping, histologic grades of sarcoma, SUV category (>5.0 or ≤5.0), and stage were not statistically significant (P>0.05).
All patients received follow-up CT scanning and FDG PET scanning. Follow-up FDG PET imaging correctly diagnosed locoregional recurrence in all 12 patients, as shown by biopsy, and distant metastases in 6 patients (Fig. 3).
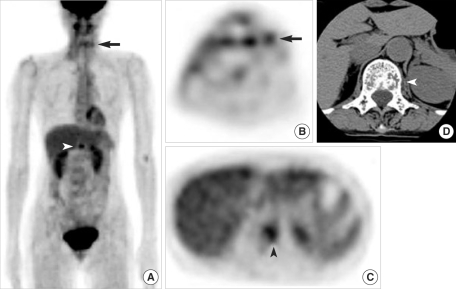
Detection of regional recurrence and distant metastasis by FDG PET. (A-C) Whole body FDG PET showing focal FDG uptakes in the left neck (arrows) and L1 vertebra (arrowheads) of a 64-yr-old melanoma patient (case no. 9). (D) Axial CT scan showing a bony metastatic lesion in the left side of the L1 vertebral body.
DISCUSSION
FDG PET is a well-established, noninvasive method for diagnostic imaging of malignancies that show increased glucose utilization compared with normal tissues (6). In patients with HNCs, FDG PET has been used clinically for tumor staging and restaging, monitoring treatment, and predicting prognosis (2-5). FDG PET has been found to be superior to conventional imaging in evaluating patients with common HNCs, including squamous cell carcinomas, lymphomas, and salivary cancers (4, 7-10). Our findings also suggest that FDG PET might be superior to conventional imaging in staging of miscellaneous cancers of the head and neck, including melanomas, basal cell carcinomas, sarcomas, and olfactory neuroblastomas. The sensitivity and specificity of FDG PET were somewhat higher than those of CT/MRI, although not significantly different from CT/MRI, due to the small numbers of patients.
Melanoma is a highly malignant tumor of the skin or, more rarely, of the mucosal surface, of the head and neck (17). Although FDG PET has been shown to detect metastases at unusual sites that are easily missed by conventional imaging modalities, the former is of limited use in patients with early-stage disease and cannot replace sentinel node biopsy (18-21). Although FDG PET was not able to detect the primary tumor site in 1 of our 6 melanoma patients, it detected cervical nodal metastases in all 7 patients with metastases, in one of whom conventional imaging failed to identify the metastases. FDG PET was shown to visualize locoregional and distant metastases in 10 patients with mucosal malignant melanoma of the head and neck (12), as well as to image and identify basal cell carcinoma, especially the nodular histologic subtype (13).
Sarcomas are a heterogeneous group of connective tissue malignancies arising in soft tissues and bone (22). Reports have shown a relationship between tumor grading and FDG avidity, and FDG PET may have a prognostic role in the management of sarcomas (23-25). In agreement with previous findings, we found that FDG PET was highly accurate in detecting primary tumors (8/9) and cervical metastases (7/9) (11).
Olfactory neuroblastomas arise from olfactory neuroepithelium, which extends from the roof of the nose to the nasal cavity, paranasal sinus, and sometimes to the intracranium (26). Tumor stage is dependent on the degree of local extension (14), and these tumors may also spread regionally to the neck, with an incidence ranging from 5% to 100% (27). To our knowledge, this study is the first to indicate that FDG PET may have a role in detecting primary and metastatic disease and in initial tumor staging of olfactory neuroblastoma.
FDG PET has been shown effective not only in identifying locoregional lesions but in detecting unsuspected distant metastases (28), and this method has been reported superior to conventional imaging modalities for detecting second primary malignancies (29). Although we found that FDG PET was accurate in detecting distant metastases and second primary cancers at initial staging and follow-up, it also identified several false positives. Therefore, to avoid false positive results, metastatic or second primary lesions showed by FDG PET in patients with these rare HNCs should be evaluated by additional diagnostic methods.
To our knowledge, this study is the largest series to date evaluating FDG PET in patients with rare HNCs (11-13). Although we attempted to minimize potential biases by comparing FDG PET and CT/MRI results with those obtained histopathologically, our study may have been limited by the inclusion of various pathologies, the small number of patients, and all initial and follow-up data. No statistical correlations between FDG uptake and the thickness of melanoma or the grade of sarcoma may result from the small number of these patients. In addition, the retrospective design may have important limitations. Another source of potential bias was that we did not routinely perform pre- and post-treatment FDG PET in all patients with rare HNCs during the study period. Nevertheless, our results indicate that FDG PET may have a role in the management of patients with rare HNCs.
In conclusion, we have investigated the clinical utility of FDG PET in patients with rare HNCs. FDG PET may have potential roles in the staging, posttreatment monitoring, and detection of distant metastases or second primary tumors, thus affecting the management of these patients. Histological grade and survival outcomes in these rare HNCs were not associated with FDG uptake, due to the small number of patients. Additional studies, in larger numbers of patients, are warranted. A more systematized policy or clinical protocol for performing follow-up PET to these patients may be also required.