Adenomatous Hyperplasia Arising from Dual Ectopic Thyroid
Article information
Abstract
Ectopic thyroid tissue is an uncommon embryologic aberration characterized by the presence of thyroid tissue in a site other than its usual pre-tracheal location. Single ectopic thyroid tissue is the most common variant, and the base of the tongue is the most frequent ectopic location. Dual ectopic thyroid is extremely rare, and only eleven cases have been reported in the English literature. Furthermore, adenomatous hyperplasia has never been reported to arise from dual ectopic thyroid. There has been only one reported case of adenomatous hyperplasia arising from a single intratracheal ectopic thyroid. We report a case of adenomatous hyperplasia arising from dual ectopic thyroid tissue that presented as a sublingual mass in a 37-yr-old woman. The diagnosis was made through pathologic examination after surgical resection. We also discuss the diagnosis and treatment of ectopic thyroid, along with a review of the literature.
INTRODUCTION
Ectopic thyroid may occur in four general locations based on the natural descent of the thyroid tissue from its embryologic starting point at the base of the tongue to its final resting position anterior to the trachea: lingual, sublingual, pre-laryngeal, and substernal (in the mediastinum) (1). The base of the tongue is the most common site of ectopic thyroid tissue, representing 90% of the cases (2). Dual ectopic thyroid refers to the simultaneous existence of two ectopic foci of thyroid tissue. Only 11 cases of dual ectopic thyroid have been reported to date (2, 4-7), and adenomatous hyperplasia has been found in only one case of single intratracheal ectopic thyroid (3). We report a rare case of dual ectopic thyroid with adenomatous hyperplasia.
CASE REPORT
A 37-yr-old female patient presented with a 6-month history of a slow growing midline submental mass. The patient did not complain of dysphagia, dysphonia, or dyspnea, but she had mild throat discomfort and cosmetic problems arising secondary to the protrusion of the submental mass. The patient had no remarkable medical, surgical, or family history. Physical examination revealed a 3×3 cm soft, movable, non-tender, submental mass, which remained in place during swallowing. Telescopic examination revealed a 2×2 cm irregularly surfaced mass covered with normal mucosa, protruding from the base of the tongue (Fig. 1).
A contrast-enhanced neck computed tomography (CT) scan showed a 2×2 cm strongly-enhancing, well-delineated, homogenous, round mass at the base of the tongue (Fig. 2A). Another 3 cm moderately-enhancing, heterogeneous, round mass was noted extending from the suprahyoid to the sublingual space (between the mylohyoid and geniohyoid muscles) (Fig. 2B). The sublingual mass seemed to reach the hyoid bone. There was no normal thyroid tissue in the pre-tracheal region (Fig. 2C). A technetium (Tc)-99m sodium pertechnetate thyroid scan showed negative uptake in the pre-tracheal area and abnormal positive uptake in the submental area (Fig. 3). However, areas of uptake in the sumental area overlapped with each other such that fine distinction was difficult.

Contrast-enhanced neck MDCT axial view: (A) It shows a well-delineated, 2×2 cm sized well enhancing homogenous round mass in base of tongue (arrow). (B) Lower section of contrast-enhanced neck MDCT axial view shows 3×3 cm sized heterogenous moderate enhancing round mass in sublingual space (arrow). (C) Absence of thyroid gland in pretracheal region is detected.

Tc-99m sodium pertechnetate thyroid scan: (A) It showed absence of normal thyroid gland in pretracheal area (arrowheads). (B) There were abnormal ectopic uptakes in submental area (arrow). Uptakes were overlapped making a fine distinction difficult.
A complete blood count with differential count and blood chemistry were in the normal range. Thyroid function tests (TFT) were normal, as follows: T3, 1.01 ng/mL (normal range, 1.9 to 0.6 ng/mL); free T4, 1.17 ng/dL (normal range, 1.8 to 0.8 ng/dL); and thyroid stimulating hormone (TSH), 2.67 µIU/mL (normal range, 5.0 to 0.25 µIU/mL).
A fine needle aspiration biopsy (FNAB) of the sublingual mass was suspicious for a thyroglossal duct cyst (TGDC). These clinical findings led us to believe there was a TGDC in the submental mass and there was ectopic thyroid at the base of the tongue.
We surgically removed the submental mass under general anesthesia using a transoral approach. We made a midline incision on the floor of the mouth between the two sides of Wharton's duct orifices and between the genioglossus muscles, and carefully dissected the tissue to reach the round mass in the sublingual space (Fig. 4A). The mass was not adhesive to the hyoid bone, and it was easily dissected from the surrounding soft tissue. Hence, we did not simultaneously resect the hyoid bone. The mass was brown-reddish and covered with normal mucosa (Fig. 4B). Histopathologic examination revealed thyroid tissue, which was highly positive on TTF-1 staining (Fig. 5A). Distended thyroid follicles were present, and were filled with colloid and manifested adenomatous hyperplasia, but were without cystic changes (Fig. 5B).
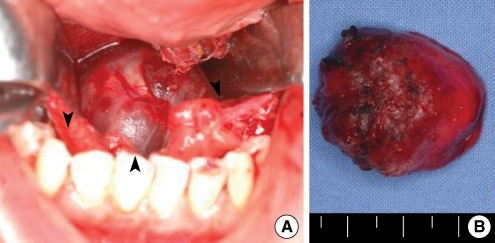
(A) An intraoperative photograph: Through a midline incision of mouth floor, we could see the reddish round mass (arrowheads). (B) Surgical specimen: it was a 3×3 cm sized firm, lobulated reddish mass covered with normal mucosa.

Histopathologic findings: (A) Specimen was strongly positive for TTF (thyroid transcription factor) stain which proves that it is from thyroid tissue (TTF-1 stain, ×100). (B) It showed large follicular cells of thyroid tissue distended with colloid material which confirms the characteristic of adenomatous hyperplasia (arrow). There was no cystic change (H and E stain, ×100).
Two months after the operation, the T3 was normal, the free T4 was decreased to 0.70 ng/mL (normal range, 1.8 to 0.8 ng/dL), and the TSH was slightly elevated to 6.04 µIU/mL (normal range, 0.25 to 5.0 µIU/mL). Four months after the operation, the TSH was 19.12 µIU/mL, so the patient was started on thyroid hormone replacement therapy with daily Synthyroxin® (50 µg, levothyroxin Na, Dalim, Seoul, Korea). During the 14-month follow-up period, no recurrence or complications was detected.
DISCUSSION
Ectopic thyroid glands are found in 1 in 100,000-300,000 persons. They are detected in 1 in 4,000-8,000 patients with thyroid disease and are 4 times more common in women than in men (1). Ectopic thyroid tissue is usually found in adolescence or in pregnancy, when the physiologic demand for hormones is increased. Patients usually present with symptoms, such as a palpable neck mass, dysphagia, dysphonia, or dyspnea, according to the location and size of the mass. In our case, the patient had a submental mass and mild throat discomfort and complained of cosmetic problems.
Dual ectopic thyroid is extremely rare, with only 11 cases reported in the English literature (2, 4-7). Bayat et al. (4) reported two cases of dual ectopic thyroid in the lingual and suprahyoid areas, similar to that seen in our case. CT scans demonstrated two equally-enhancing homogenous masses. The masses showed a degree and pattern of enhancement resembling those of orthotopic thyroid gland; they were subsequently treated with thyroxine therapy. However, in our case, the submental mass was heterogeneous. This variation from orthotopic thyroid gland tissue made the diagnosis difficult.
A neck ultrasound (US) examination, neck CT scan, thyroid scan, and thyroid uptake test are all valuable modalities for forming a differential diagnosis (8). US examination plays a role in differentiating cystic and solid masses. On CT scan, thyroid tissue has a characteristically high enhancement secondary to its great vascularity. A thyroid scan is the most definitive non-invasive technique for establishing the diagnosis of ectopic thyroid (4). As a functional imaging tool, thyroid scanning enables distinction of ectopic thyroid from other oral cavity and cervical masses. FNAB may exclude malignancy or other pathologic changes in heterogeneous tissue. The potential for malignant degeneration in ectopic and eutopic thyroid tissue is similar to that seen in the dominant subtype of papillary carcinoma (7).
The differential diagnosis of an ectopic thyroid gland with other neck masses is important because inadvertent removal of ectopic thyroid tissue may result in permanent hypothyroidism. Several cases have been reported in which ectopic thyroid tissue was mistaken for TGDC (9-11). The differential diagnosis of a neck mass should include TGDC, dermoid cyst, epidermoid cyst, lipoma, and lymphadenitis.
Asymptomatic and euthyroid state patients do not need to be treated. Patients with symptoms, such as dysphagia or dyspnea, or cosmetic problems, need to be treated. First-line treatment for ectopic thyroid tissue is medical therapy with thyroxine, which suppresses the TSH level and leads to regression of the ectopic thyroid tissue (8, 12). Surgical resection is considered in patients who fail medical treatment, in those who are suspicious of malignancy, and in those who have cosmetic problems (6). However, 70-90% of patients have no functioning thyroid tissue besides that found in the ectopic location, so extreme caution must be exercised; removal of the only functioning thyroid tissue will result in permanent hypothyroidism (8, 10). In our case, it would have been better to begin with medical treatment. However, given the rarity of dual ectopic thyroid, it was hard for us to consider the possibility that the submental mass represented an ectopic thyroid. The submental mass enhanced differently from normal thyroid tissue on CT, and FNA was suspicious of TGDC. Hence, we felt tissue confirmation was needed.
The final histopathologic diagnosis of the submental mass was adenomatous hyperplasia in ectopic thyroid tissue. Adenomatous hyperplasia in suprahyoid ectopic thyroid coexisting with lingual ectopic thyroid is extremely rare. A literature search demonstrated only one case report of a single ectopic thyroid with adenomatous hyperplasia (3). Sung et al. (3) reported a 33-yr-old pregnant woman presenting with a single intratracheal ectopic thyroid and orthotopic thyroid with adenomatous hyperplasia. In that case, the ectopic thyroid was connected with the orthotopic thyroid. The patient was treated through segmental resection and anastomosis of the trachea.
In summary, we have described a rare case of dual ectopic thyroid with adenomatous hyperplasia. Ectopic thyroid tissue is an extremely rare entity and poses diagnostic and treatment problems. CT scan revealed a highly enhancing lingual mass and a heterogeneous, moderately enhancing sublingual mass. FNAB of the sublingual mass was suspicious of TGDC. However, a surgically removed sublingual mass was proved to be ectopic thyroid tissue with adenomatous hyperplasia, which is extremely rare. After surgical resection, increased TSH levels were controlled with Synthyroxin® (dalim, Seoul, Korea).
