Diagnosis of Obstructive Sleep Apnea in Adults Using the Cardiopulmonary Coupling-Derived Software-Generated Apnea-Hypopnea Index
Article information
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction and apneic spells during sleep [1]. The standard diagnostic modality for OSA is polysomnography (PSG), and the presence and severity of OSA are determined using the apnea-hypopnea index (AHI) [2]. AHI values are derived from assessments of respiratory flow, oxygen desaturation, and arousal during sleep. Thus, a great amount of medical facilities, resources, and efforts are needed to perform PSG, which has spurred many physicians to attempt to develop simpler diagnostic modalities for OSA. With this goal in mind, we assessed sleep quality and stability using cardiopulmonary coupling (CPC) analysis [3,4]. Electrocardiogram (ECG)-based CPC analysis reflects sleep stability using cardiac regulation and respiratory variation [5]. In these studies, we found that the parameters related to sleep quality and stability generated from CPC analysis were significantly correlated with the AHI. Recently, Hilmisson et al. [6] published an article on sleep apnea diagnosis using a software-generated AHI (sAHI) derived from CPC analysis in children. They reported that the sAHI was comparable to the manual scoring of AHI from in-laboratory PSG studies and was effective for assessing OSA severity in children. Thus, in this study, we investigated the diagnostic value of the sAHI in adults using a cloud-based software technology, SleepImage (MyCardio, Denver, CO, USA).
We reviewed the medical records of 194 adult patients (age, 18–72 years) with sleep-disordered breathing who underwent full-night in-laboratory PSG and CPC analysis concurrently between 2007 and 2013. We excluded patients who had insufficient sleep (total sleep time <6 hours), neuroactive drug usage, or concurrent significant medical comorbidities. This study protocol was reviewed and approved by the Institutional Ethics Committee at Korea University Ansan Hospital (IRB No. 2020-AS0041).
Overnight physician-attended standard PSG was carried out using an Alice4 (Respironics, Murrysville, PA, USA) device using the standard neurophysiologic and respiratory signals recommended by the American Academy of Sleep Medicine (AASM). PSG data were manually scored by well-trained sleep technicians and additionally reviewed by certified sleep physicians according to the recommended AASM criteria [2]. We defined OSA as an AHI of ≥5 events/hr.
All study participants underwent CPC analysis using the RemLogic 2.0 CPC analyzer (Embla Systems Inc., San Carlos, CA, USA) during in-laboratory full-night PSG. The CPC data were generated by QRS complex amplitude variations from a single-lead ECG channel, and various parameters were obtained and calculated automatically according to the frequency [5,7,8]. The sAHI value was automatically calculated from the analysis of CPC parameters, and non-hypoxic events were calculated from the frequency of coupling during subtypes of elevated low frequency coupling (e-LFC; narrowband–e-LFC [e-LFCNB] or broadband–e-LFC [e-LFCBB]). The total number of events was divided by the total sleep time and represented as the sAHI [6]. Statistical analysis was carried out using IBM SPSS ver. 21 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
The Spearman correlation coefficient showed that the sAHI was significantly positively correlated with the AHI (r=0.973, P<0.001) (Fig. 1). In addition, Bland-Altman analysis found that the AHI and sAHI values showed good agreement and correlations (Supplementary Fig. 1). The mean difference was −2.00 events/hr and the upper and lower limits of agreement were 10.64 and −14.64, with only 3.09% (6/194) of cases outside of this limit.
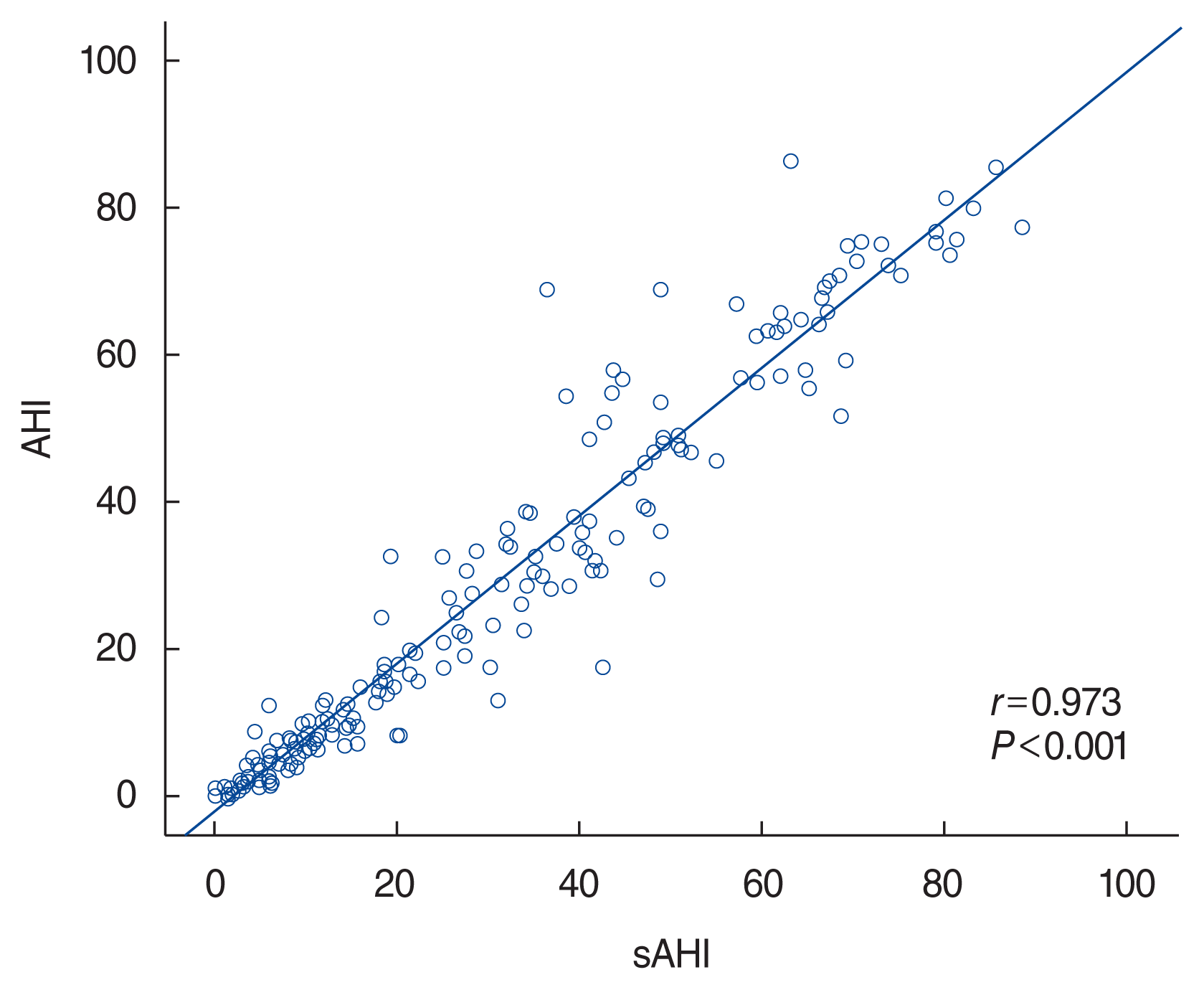
Correlation coefficients between apnea-hypopnea index (AHI) and software-generated AHI (sAHI) values. The Spearman correlation method was used to evaluate the relationship between both the parameters, and the degree of correlation (Spearman’s rho; r) was calculated.
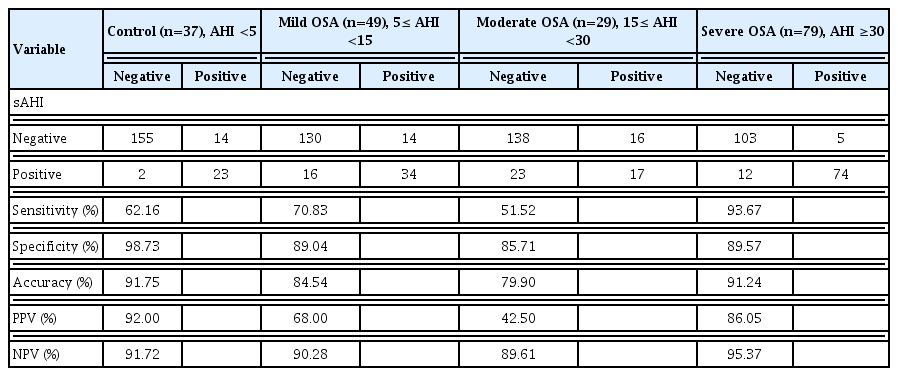
The diagnostic value of the sAHI was compared to the standard parameters used to calculate the AHI, and the results are presented in Table 1. The sensitivity and specificity according to OSA severity were 62.16% and 98.73% for the controls, 70.83% and 89.04% for mild OSA, 51.52% and 85.71% for moderate OSA, and 93.67% and 89.57% for severe OSA, respectively. Receiver operating characteristic analysis for the diagnosis of OSA based on the sAHI cutoff point of 5.0 events/hr revealed an area under the curve of 0.987 (Supplementary Fig. 2). The diagnostic value was highest for patients with severe OSA. The optimal cutoff values for the diagnosis of OSA using the sAHI were 9.1/hr (sensitivity, 100%; specificity, 93.63%; 95% confidence interval, 88.6%–96.9%).
In this study, we found that the CPC-based sAHI was significantly correlated with the manually-scored AHI. Furthermore, sAHI values can be used to diagnose OSA and to assess its severity with some adjustments. As mentioned above, we previously reported that CPC values closely reflected sleep quality and stability [3,4]. Recently, a cloud-based software technology, SleepImage, has been approved by the Food and Drug Administration [6]. Thus, we could generate sAHI values and assess the diagnostic value of the sAHI in our adult population. Hilmisson et al. [6] recently published an article on the diagnostic value of the sAHI in the pediatric population, and reported strong agreement between the sAHI and AHI in children. In this study, we also found similar results in adult patients with OSA. However, we found that the diagnosis and assessment of OSA using the same AHI values as generated from PSG yielded relatively low sensitivity, except for patients with severe OSA. Therefore, it was necessary to suggest a new sAHI cutoff value for OSA diagnosis, and we found that a sAHI value of 9.1/hr was optimal, yielding a diagnostic accuracy of 100% sensitivity and 93.63% specificity.
This study compared respiratory parameters calculated using PSG and CPC. The CPC analysis could be performed using either ECG or photoplethysmography with a portable device [6]; therefore, we consider that this modality might be more convenient than the conventional method. With this convenient diagnostic modality, we can perform multiple tests per day in a patient-friendly sleep environment, such as the patient’s own home. Therefore, in light of these advantages, we believe that CPC can be actively used for the diagnosis of sleep-disordered breathing.
In our study, the control and moderate OSA groups contained somewhat fewer patients than the severe OSA group, which may have affected the findings regarding diagnostic accuracy in our study groups.
In conclusion, we suggest that this simple diagnostic modality using CPC might be an alternative to standard PSG for OSA diagnosis in adult patients for whom standard PSG is not feasible.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: SHL. Data curation: MYS, JY, SJH. Funding acquisition: MYS. Methodology: SHL. Writing–original draft: MYS. Writing–review & editing: SHL, MYS.
ACKNOWLEDGMENTS
Mr. Hugi Hilmisson, the Director of Research & Development at SleepImage®, generated the sAHI values from our CPC data.
This study was supported by National Research Foundation of Korea (NRF) grant funded by Korean government (Ministry of Education) (NRF-2020R1I1A1A01063604 to Min Young Seo).
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.21053/ceo.2020.01984.