Guideline for the Surgical Management of Locally Invasive Differentiated Thyroid Cancer From the Korean Society of Head and Neck Surgery
Article information
Abstract
The aim of this study was to develop evidence-based recommendations for determining the surgical extent in patients with locally invasive differentiated thyroid cancer (DTC). Locally invasive DTC with gross extrathyroidal extension invading surrounding anatomical structures may lead to several functional deficits and poor oncological outcomes. At present, the optimal extent of surgery in locally invasive DTC remains a matter of debate, and there are no adequate guidelines. On October 8, 2021, four experts searched the PubMed, Embase, and Cochrane Library databases; the identified papers were reviewed by 39 experts in thyroid and head and neck surgery. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence, and to develop and report recommendations. The strength of a recommendation reflects the confidence of a guideline panel that the desirable effects of an intervention outweigh any undesirable effects, across all patients for whom the recommendation is applicable. After completing the draft guidelines, Delphi questionnaires were completed by members of the Korean Society of Head and Neck Surgery. Twenty-seven evidence-based recommendations were made for several factors, including the preoperative workup; surgical extent of thyroidectomy; surgery for cancer invading the strap muscles, recurrent laryngeal nerve, laryngeal framework, trachea, or esophagus; and surgery for patients with central and lateral cervical lymph node involvement. Evidence-based guidelines were devised to help clinicians make safer and more efficient clinical decisions for the optimal surgical treatment of patients with locally invasive DTC.
INTRODUCTION
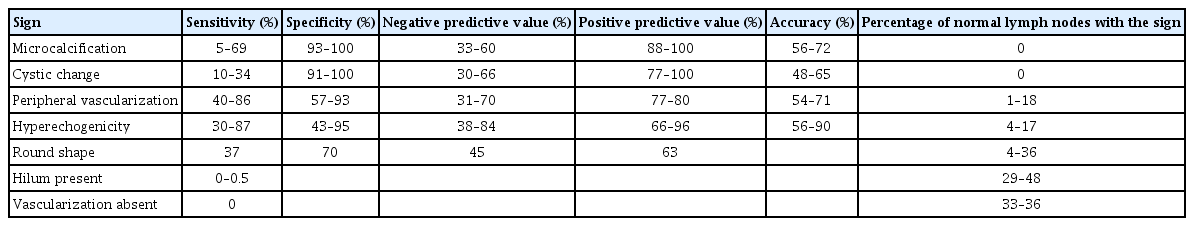
Differentiated thyroid cancer (DTC) with gross extrathyroidal extension (ETE) invading the surrounding anatomical structures occurs in 5%–15% of cases. The adjacent structures most frequently invaded by thyroid cancer are the strap muscles and recurrent laryngeal nerve (RLN), followed by the upper trachea, laryngotracheal junction, pharyngoesophageal conduit, and major vessels [1-3]. As critical anatomical structures are in close proximity to the outside of the thyroid gland, gross ETE of thyroid cancer can cause functional deficits, such as voice changes, acute upper airway obstruction, and dysphagia (Fig. 1).
Critical anatomical structures adjacent to the thyroid gland: trachea, larynx, esophagus, and recurrent laryngeal nerve.
A tumor of any size with gross ETE invading the subcutaneous soft tissues, larynx, trachea, esophagus, or RLN is defined as T4a disease, while gross ETE invading only the strap muscles is classified as T3b disease, according to the eighth American Joint Committee on Cancer (AJCC) cancer staging system [4]. Although the prognosis of DTC is generally good, locally invasive DTC may lead to poor oncological outcomes, with higher rates of recurrence, distant metastasis, and mortality [5-14]. Hotomi et al. [14] suggested that the degree and site of invasion determine the prognosis in patients with locally invasive DTC. Invasion of the mucosa of the trachea or esophagus, as well as preoperative RLN palsy, had poorer prognoses. Without mucosal invasion of the trachea and esophagus, the prognosis is better after shaving tumors off laryngotracheal structures, resecting only the muscular layer of the esophagus [14].
Although the primary goal of surgery is complete eradication of all cancer tissues with safe margins, this is not always achievable in cases of locally invasive DTC because radical resection is challenging and critical anatomical structures may be damaged (which can cause serious functional deficits and complications). Some advanced cases require aggressive resection of extrathyroidal tissues. Options for achieving acceptable disease-specific survival include incomplete removal of the primary tumor in cases of microscopic or macroscopic residual disease, and postoperative radioactive iodine ablation with thyrotropin suppression [15,16]. At present, the extent of surgery in patients with locally invasive DTC remains a matter of debate; randomized controlled trials and adequate guidelines are lacking. The existing guidelines for thyroid cancer treatment contain mainly information on the scope of surgery and the general diagnosis and treatment policies, but lack details regarding decision-making before, during, and after surgery, especially for locally invasive DTC [17-19]. Recently published guidelines in the United States include details for surgical treatment, but these guidelines do not mention surgical treatment for locally invasive DTC [20]. There have been few large-scale clinical studies or systematic reviews on locally invasive DTC. Therefore, surgeons have to rely on their own or institutional experience for decision-making regarding the surgical treatment of locally invasive DTC, and the clinical outcomes therefore differ among surgeons and institutions.
These guidelines are intended to help clinicians make clinical decisions more safely and efficiently for patients with locally invasive DTC. The final goal is to extend patients’ life expectancy and enhance their quality of life, as well as to improve public health by developing standards of surgical management for locally invasive DTC.
MATERIALS AND METHODS
Target population
The guidelines deal with papillary and follicular carcinoma only, and not medullary thyroid cancer or anaplastic carcinoma. These guidelines target locally invasive DTC that can be surgically resected with curative intent, including the following: tumors >4 cm limited to the thyroid (T3a), gross ETE invading only the strap muscles (sternohyoid, sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any size (T3b), and gross ETE invading the subcutaneous soft tissues, larynx, trachea, esophagus, or RLN from a tumor of any size (T4a).
Organization of the committee
The guidelines were developed by the Guideline Committee of the Korean Society of Head and Neck Surgery (KSHNS). A task force was appointed consisting of six teams comprising 39 experts. The task force chairman and vice-chairman were appointed by the President of the KSHNS with the approval of the committee. The committee members were supported by experts in literature searches, systematic reviews, and the development and establishment of guidelines. The committee members participated in conference calls to review and evaluate each major stage of guideline development at regularly scheduled online or face-to-face meetings.
Literature search and quality assessment
A literature search was conducted in the PubMed, Embase, and Cochrane Library databases by four literature search experts on October 8, 2021. As search terms, MeSH terms were used in the PubMed and Cochrane Library database searches, and Emtree terms were used in the Embase database search. Related text words were also added. The search terms were combined by Boolean operators (AND, OR, NOT). We applied the following limits during the search: search fields—title, abstract, keywords; publication type—article, review, article in press; species—human. The search was not limited by publication year. After reviewing the title, unrelated documents were excluded. The remaining selected documents were reviewed independently by two committee members, who decided whether they should be excluded or included. The search string used for each key question and the number of documents retrieved are shown in Supplementary Table 1 and Supplementary Fig. 1.
Quality of the literature and evidence, and the grading of recommendations
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which represents an international standard for making transparent recommendations, was used to assess the quality of evidence and develop and report recommendations. Guideline committee members were trained on the process prior to the guideline meeting via a combination of online training modules, publications, and presentations. The Risk of Bias Assessment Tool for Nonrandomized Studies was used to test non-randomized control trials and to assess the quality of observational studies, and A Measurement Tool to Assess the Methodological Quality of Systematic Reviews was used to evaluate systematic reviews and meta-analyses [21,22]. The quality of evidence was accessed independently by two committee members, and in cases of disagreement, the members reached a consensus by discussion. GRADE categorizes the quality of evidence as high, moderate, low, or very low. The quality of the available evidence, and judgments that bear on the ratings of the level and strength of the evidence are summarized in Supplementary Material 1.
The strength of a recommendation reflects the extent to which a guideline panel is confident that the desirable effects of an intervention outweigh its undesirable effects, or vice versa, across all patients for whom the recommendation is intended. According to GRADE, the definitions of strong and conditional recommendations are as follows: a strong recommendation indicates that the panel is confident that the desirable effects of adherence to a recommendation outweigh the undesirable effects; a conditional recommendation indicates that the panel concludes that the desirable effects of adherence to a recommendation probably outweigh the undesirable effects, but is not confident. No recommendation is given if the panel is reluctant to make a recommendation for or against a particular management strategy as the confidence in the effect estimate is too low or the trade-offs are so closely balanced, or two options have very different undesirable consequences. The direction and strength of the recommendations were determined considering the balance between desirable and undesirable outcomes (trade-offs), taking into account the quality of evidence, confidence in the values and preferences and their variability, and resource use (Supplementary Material 1).
The Delphi method was used to establish consensus. The panel consisting of KSHNS members was asked to review the recommendations by email. In total, 27 recommendations were distributed to all members, and they were asked to give one of the following responses to each recommendation: “fully agree,” “agree,” “neither agree nor disagree,” “disagree,” or “totally disagree.” A recommendation was finally accepted if more than two-thirds of the panel members gave a response of “fully agree” or “agree.” After the first round, 58 surgeons answered the first Delphi question. Consensus was achieved for 25 of the 27 recommendations. Recommendations that failed to reach agreement by two-thirds of the panel were modified according to feedback from experts and redistributed to second-round offline panels. In the second round, 32 experts used the same voting methods described in the first round, but in an off-line meeting. The final responses were analyzed as described in the first round, and two recommendations were resolved by consensus among the entire panel of experts. These 27 recommendations were reorganized and combined into the 19 recommendations discussed below.
GUIDELINES FOR THE SURGICAL MANAGEMENT OF LOCALLY INVASIVE DIFFERENTIATED THYROID CANCER
The organization of the Guidelines for the Surgical Management of Locally Invasive Differentiated Thyroid Cancer are summarized in Table 1.
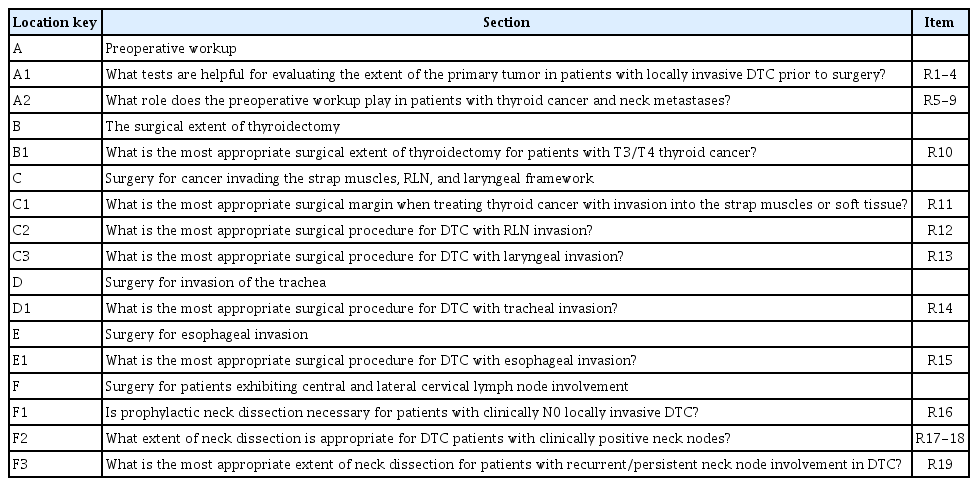
Organization of the KSTHNS Guidelines for the Surgical Management of Locally Invasive Differentiated Thyroid Cancer
A. Preoperative workup
A1. What tests are helpful for evaluating the extent of the primary tumor in patients with locally invasive DTC prior to surgery?
A preoperative evaluation of the extent of the primary tumor in patients with suspected or confirmed DTC provides important information for planning the extent of surgery. An appropriate evaluation of the primary lesion allows complete resection, which provides accurate staging and risk stratification of the cancer, thereby reducing the risk of persistent or recurrent disease. In addition, appropriate evaluation before surgery also minimizes surgery-related morbidity by avoiding the unnecessary resection of major structures around the thyroid gland. Therefore, detailed preoperative staging of the primary lesion is essential and helps provide appropriate counseling to patients regarding the surgical plan, complications expected after surgery, and additional postoperative treatments.
Recommendation 1 Routine preoperative evaluation of vocal fold movements or laryngeal structures using laryngoscopy is recommended to determine the extent of disease in patients with locally inva-sive DTC.
(Strong recommendation/low-quality evidence)
Laryngoscopy, which is familiar to head and neck surgeons, is easily applied before thyroid surgery to evaluate vocal fold movement and the laryngeal invasion of thyroid tumors, and it provides the most intuitive and visual information about vocal fold movement and the laryngeal mucosa. Although invasion of the RLN and laryngeal structures by DTC is relatively rare, it is an important issue associated with negative impacts on prognosis and functional disabilities of the airway, voice, and swallowing, because resection of laryngeal structures or the RLN may be required [23-25].
Recommendation 2 Preoperative evaluation of the primary tumor using ultraso-nography is recommended in patients undergoing thyroid surgery for locally invasive DTC.
(Strong recommendation/moderate-quality evidence)
Ultrasonography (US) provides useful information for the diagnosis of thyroid nodules, the determination of preoperative staging, and the detection of DTC recurrence [17]. Therefore, a detailed evaluation of the extent of the primary tumor should be performed in all patients with suspected locally invasive DTC. Tumor size >4 cm (T3a), ETE into the strap muscles (T3b), and ETE into major structures of the neck (T4) are significant findings in the preoperative staging of locally invasive DTC, which can be detected on US evaluation [26]. US was reported to show an accuracy of 81.8% for determining T stage and a sensitivity of 77.8% for determining ETE into the strap or sternocleidomastoid muscles [27]. However, the diagnostic criteria of ETE are subjective and dependent on the physician, leading to a wide range of diagnostic sensitivity, specificity, and accuracy values [28-31]. Tomoda et al. [32] reported that US was useful a screening tool for evaluating tracheal invasion by primary thyroid tumors, with an accuracy of 93%, sensitivity of 91%, specificity of 93%, positive predictive value (PPV) of 25%, and negative predictive value (NPV) of 99%. All diagnostic values except PPV were higher than those of magnetic resonance imaging (MRI) [32]. As it is difficult to evaluate substernal thyroid tumors, mediastinal lymph node metastasis, and the intraluminal extent of laryngotracheal invasion, other imaging studies or alternative methods should be considered to evaluate the extent of disease in such cases.
Recommendation 3 Cross-sectional imaging (computed tomography [CT] or MRI) is helpful for evaluating anatomical relationships be-tween the tumor and surrounding visceral structures in lo-cally invasive DTC.
(Strong recommendation/low-quality evidence)
CT or MRI may provide important information in patients with locally invasive DTC. Such imaging studies are useful for evaluating the anatomical relationships between thyroid tumors and major cervical structures, including the larynx, trachea, esophagus, and major vessels [33,34]. While US has limitations in evaluating lesions behind the sternum, CT and MRI have advantages in evaluating the substernal extension of thyroid tumors, cervical and mediastinal lymph node metastases, and tracheal involvement [35]. MRI may also be clinically useful in determining whether to resect a functioning RLN or not before surgery in cases with suspected RLN invasion by primary thyroid tumors [36]. Therefore, cross-sectional imaging studies should be considered for patients with locally invasive DTC with suspected visceral component invasion or cervical lymph node metastasis.
Recommendation 4 Bronchoscopy/esophagoscopy evaluation may provide ancil-lary information about the extent of disease in patients with suspected tracheal or esophageal invasion.
(Conditional recommendation/low-quality evidence)
Laryngotracheal invasion is observed in 35%–60% of patients with locally invasive thyroid cancer, and an accurate evaluation of these findings before surgery is important for the establishment of an appropriate surgical plan. Either a shaving operation or extensive resection can be selected depending on the degree of tracheal invasion [2,37,38]. Although CT and MRI are useful in assessing the intraluminal involvement of thyroid cancer, they have limitations in precisely determining the depth and length of laryngotracheal invasion [39-41]. In addition, these imaging tools have limitations in determining the depth of invasion into the esophagus by primary thyroid malignancies [42,43]. Therefore, in patients with suspected laryngeal, tracheal, or esophageal invasion, when it is difficult to evaluate the depth and length of invasion of thyroid cancer by CT or MRI, bronchoscopy or esophagoscopy can be used to evaluate the extent of invasion for surgical planning.
A2. What role does the preoperative workup play in patients with thyroid cancer and neck metastases?
Metastasis of cervical lymph nodes may be identified in 20%–50% of patients diagnosed with papillary thyroid carcinoma (PTC). As cervical lymph node metastasis increases the risk of local or regional recurrence, a preoperative workup of the central or lateral neck is important to select an optimal surgical strategy [44].
Recommendation 5 Diagnostic neck US should be performed in patients diag-nosed with locally invasive thyroid cancer to detect central or lateral neck metastasis.
(Strong recommendation/moderate-quality evidence)
High-resolution US is considered the method of choice for the detection and diagnosis of cervical lymph node metastasis in patients with DTC. The sensitivity of US imaging is reported to be higher for detecting lateral than central lymph node metastasis [44,45]. Choi et al. [46] reported that the overall accuracy of US for N categorization was 59%, and that it had accuracies of 33.3% and 85.1% for N1a and N1b categorization, respectively. A recent meta-analysis including 4,014 patients reported that US had pooled sensitivity and specificity values of 33% and 93%, respectively, for detecting central lymph node metastasis and 70% and 84%, respectively, for detecting lateral neck metastasis [47]. The US features suggesting lymph node metastasis are focal or diffuse hyperechogenicity, calcification, cystic changes, round shape (longitudinal/transverse ratio <2) with loss of hilar echogenicity, and abnormal vascular patterns [44-46,48].
Recommendation 6 US-guided fine needle aspiration of suspicious lymph nodes should be considered to confirm the presence of metastasis.
(Conditional recommendation/moderate-quality evidence)
US-guided fine needle aspiration (FNA) has a high sensitivity and specificity and has traditionally been used for the diagnosis of cervical neck lymph node metastasis, with a sensitivity of 96%, specificity of 73%, PPV of 99%, NPV of 27%, and accuracy of 95% [49]. The sensitivity and specificity of US-guided FNA may be restricted to some extent by various factors, such as suspicious or nondiagnostic cytology, metastases from extrathyroidal malignancies, and interference by inflammatory lymphadenopathies [50].
Recommendation 7 A thyroglobulin assay using FNA (FNA-Tg) may be useful to identify metastasis in selected cases with suspicious cervical lymph nodes. The combination of FNA cytology and FNA-Tg may show better diagnostic performance than cytology alone.
(Conditional recommendation/moderate-quality evidence)
Preoperative US-guided FNA is recommended for suspicious lymph nodes if the outcome is expected to change the management [17,51]. However, small lymph nodes may be difficult to aspirate, and the complex cytological features of lymph nodes with cystic changes may lead to a false-negative rate of 6%–8% [51]. Recent systematic reviews [51,52] suggested that a FNA-Tg has a high accuracy for detecting nodal metastasis in DTC. Xu et al. [52] reported that the diagnostic value for detecting lymph node metastasis ranked as follows: combination of FNA and FNA-Tg>FNA-Tg>FNA alone. FNA-Tg increased the diagnostic sensitivity among nondiagnostic FNA outcomes, particularly in lymph nodes with cystic changes [53,54]. False positive results due to contamination should be considered in patients with an intact thyroid gland [53]. Konca Degertekin et al. [50] reported that cutoff values of 1 ng/mL and 10 ng/mL yielded accuracies of 94.1% and 88.2%, respectively. Due to the differences in Tg assay methods among studies, there is no consensus on the most appropriate Tg cutoff value, and the criteria require standardization [51].
Recommendation 8 CT with contrast enhancement is recommended to evaluate central and lateral metastatic lymph nodes in patients with locally invasive thyroid cancer.
(Strong recommendation/moderate-quality evidence)
Contrast-enhanced CT is recommended as an adjunct to US in patients with clinically suspected lymph node metastasis [17,55-57]. CT may have a complementary role as US is operator-dependent and is limited in its ability to examine deep anatomical structures [55]. Lee et al. [56] reported that the combination of CT and US improved surgical planning by enhancing the sensitivity of detecting lymph node metastases overlooked by US alone in cases of metastasis in the retropharyngeal or superior mediastinal compartment. A systematic review and meta-analysis [55] including 1,691 patients showed that CT had a sensitivity of 62% and specificity of 87% for diagnosing cervical lymph node metastasis when using level-by-level analysis. The sensitivity of CT combined with US (69%) was significantly higher than that of US alone (51%).
Recommendation 9 Preoperative F-18 fluorodeoxyglucose positron emission to-mography (18FDG-PET) or PET/CT may play a complemen-tary role in detecting regional or distant metastasis in pa-tients with advanced thyroid cancer.
(Conditional recommendation/low-quality evidence)
18FDG-PET/CT may be useful in some patients with neck or mediastinal involvement of thyroid cancer. However, due to the relatively low sensitivity of detecting cervical lymph node metastasis, routine preoperative 18FDG-PET/CT examinations are not recommended [17]. In fact, its utility as an initial examination to detect lymph node metastasis in patients with thyroid cancer has not been fully evaluated, and Kim et al. [58] reported that PET positivity was unrelated to lymph node metastasis. A recent meta-analysis [59] reported that 18FDG-PET or PET/CT showed no significant differences in sensitivity, specificity, PPV, NPV, or accuracy compared with CT or US. Nonetheless, due to the FDG avidity or SUVmax, this modality may play a complementary diagnostic role along with US and CT, with clinical value for detecting lymph node metastasis regardless of size, calcification, or cystic changes [59].
B. The surgical extent of thyroidectomy
B1. What is the most appropriate surgical extent of thyroidectomy for patients with T3/T4 thyroid cancer?
Recommendation 10
(A) If the tumor size is ≥4 cm, (near) total thyroidectomy should be considered regardless of infiltration. (Conditional recommendation/moderate-quality evidence)
(B) If gross ETE invading the strap muscle is seen in thyroid cancer, (near) total thyroidectomy can be considered. (Conditional recommendation/moderate-quality evidence)
(C) If gross ETE into major neck structures, such as subcuta-neous soft tissues or the larynx, trachea, esophagus, or RLN is seen, (near) total thyroidectomy should be con-sidered. (Conditional recommendation/low-quality evidence)
In the AJCC staging system, eighth edition, T3 DTC is divided into T3a (tumor size >4 cm) and T3b (tumor with gross ETE invading only the strap muscles), and microscopic ETE is excluded from T3. Practically, total thyroidectomy is generally accepted for tumors >4 cm, and only a few studies have investigated this issue in tumors <4 cm or ≤4 cm in diameter [60-62]. Several reports have compared oncological outcomes between thyroid lobectomy and total thyroidectomy to investigate the most appropriate surgical extent for tumors 2–4 cm in size. In an analysis of 33,816 DTC patients, Rajjoub et al. [60] reported that adjusted analysis showed total thyroidectomy was associated with improved survival in patients with tumors 2.0–3.9 cm in diameter (P =0.03) compared to those with conventional papillary thyroid cancer. Based on Surveillance, Epidemiology, and Results (SEER) data from 23,605 patients, Barney et al. [61] reported that total thyroidectomy resulted in better overall survival compared with lobectomy in patients with DTC <4 cm. In an analysis of 52,173 patients, Bilimoria et al. [62] reported better local recurrence and overall survival rates after total thyroidectomy in patients with papillary thyroid cancer >1 cm than after lobectomy. Generally, total thyroidectomy has shown better results than those of thyroid lobectomy. The 2015 American Thyroid Association (ATA) management guidelines for adult patients with thyroid nodules and DTC [17] also endorsed total thyroidectomy as the primary initial surgical treatment option for all DTC >4 cm or with gross ETE.
In an analysis of 63,315 patients with DTC, Xiang et al. [63] reported a poorer cancer-specific survival rate in patients with gross ETE invading the strap muscles (T3b) than in those with no ETE or minimal ETE, but a better rate than in patients with T4 disease. However, the impact of gross ETE invading only the strap muscles, which is the most closely adjacent structure, on prognosis is still not clear due to sparse and conflicting data. In an analysis of 3,104 DTC patients, Song et al. [64] reported that the disease-specific survival rate of patients with small tumors (≤4 cm) with gross ETE into the strap muscles only was not different from that of those with T2 disease, but better than that of those with T3a disease. In an analysis of 3,174 DTC patients, Park et al. [65] reported no difference in the 10-year cancer-specific survival rate among patients with gross ETE into the strap muscles only, those without ETE, and those with microscopic ETE. Based on the above studies, even if there is gross ETE invading only the strap muscles, thyroid lobectomy could be an option in cases with a small tumor and no other risk factors, such as evidence of lymphatic or distant metastasis. However, further evidence is needed to provide generalized recommendations to reduce the surgical extent.
Although there is no consensus regarding the application of total thyroidectomy versus thyroid lobectomy in select situations, considering the literature on the association of larger tumor size and gross ETE with poor prognosis and the possibility of postoperative radioactive iodine ablation therapy, (near) total thyroidectomy is generally suggested in cases of T3 disease [63, 64,66-70]. Gross ETE beyond the strap muscles is considered T4a according to the eighth edition of the AJCC staging system. Unfortunately, there have been no reports in English regarding oncological outcomes according to the extent of thyroidectomy in T4a disease. Total thyroidectomy has been widely accepted as appropriate for patients with T4a disease.
C. Surgery for cancer invading the strap muscles, RLN, and laryngeal framework
C1. What is the most appropriate surgical margin when treating thyroid cancer with invasion into the strap muscles or soft tissue?
Recommendation 11 If invasion of the strap muscles is confirmed by inspection or palpation with/without frozen biopsy during surgery, com-plete resection of the invaded tissue with adequate safety margins is recommended.
(Conditional recommendation/low-quality evidence)
As mentioned above in “A. Preoperative workup,” the role of imaging studies to evaluate invasion into the strap muscles or soft tissue in DTC is a matter of debate [30,71,72]. Therefore, it is often challenging to determine the area of the strap muscles or soft tissue resection preoperatively. Inspection and palpation during surgery are the simplest and most widely accepted methods to confirm strap muscle invasion. The diagnostic role of frozen biopsy to confirm ETE in DTC has not been fully investigated. Park et al. [73] reported that frozen biopsy was superior to US for identifying ETE, with a sensitivity, specificity, PPV, and NPV of 66%, 99%, 98%, and 87%, respectively.
There is still no evidence regarding the most appropriate safety margin in cases of strap muscle or soft tissue invasion. Most surgeons decide on the adequate margin based on their own experience based on inspection and palpation, with or without frozen biopsy. At this point, surgeons should take into consideration that excessive strap muscle resection may affect postoperative voice outcomes and swallowing function when determining the resection area [74,75]. In addition, surgeons should also consider that the strap muscles could be used for reconstruction of laryngeal and tracheal defects in the case of disease recurrence and progression. Therefore, further studies are needed to refine the balance between oncological safety and functional preservation when determining surgical margins.
C2. What is the most appropriate surgical procedure for DTC with RLN invasion?
Recommendation 12
(A) Nerve-preserving procedures can be attempted if the DTC invades the RLN while vocal fold movement is re-tained. (Conditional recommendation/low-quality evidence)
(B) The RLN may be resected if the DTC invades the RLN and vocal fold palsy is identified. (Conditional recommendation/low-quality evidence)
RLN injury is a major complication of thyroid cancer surgery and may also be associated with voice changes and respiratory complications due to difficulty with breathing and aspiration. In cases of DTC invading the RLN, the surgical method could differ depending on the presence or absence of vocal cord palsy on preoperative evaluation. Typically, patients with vocal cord palsy may show a rough and breathy voice quality. A preoperative laryngoscopic assessment of vocal cord mobility by flexible laryngoscopy or stroboscopy and neck US can be performed to confirm vocal cord movement. If vocal cord mobility is confirmed preoperatively, and thus the RLN is regarded as functional, the RLN may be treated conservatively using the shaving technique. At this point, the surgeon should comprehensively consider the following factors to determine the surgical technique: the occupation of the patient (especially professional voice users), degree of nerve invasion (extent of encircling), general health status, and function of the contralateral RLN. RLN function was preserved in >80% of patients with nearly normal phonatory function 1 year after applying the nerve-shaving technique [76,77]. There were no differences in the local recurrence or survival of patients with DTC invading the RLN who underwent nerve-preserving procedures versus nerve resection. The overall recurrence rates were 44% for the nerve-preserving procedure and 35% for nerve resection (P =0.5691) [78]. The 10-year local disease-free survival rate and 10-year disease-free survival rate were similar between the shaving resection and complete resection groups (76.9% vs. 79.3%, P =0.612 and 73.0% vs. 68.5%, P =0.382, respectively) [15]. Inconsistent results have been reported regarding whether patients with DTC invading the RLN have poor oncological outcomes [79-81]. Intraoperative nerve monitoring is now widely performed during thyroid surgery and has been shown to be useful for predicting postoperative RLN function in cases in which the RLN is preoperatively paralyzed or invaded by a tumor [82].
If vocal cord paralysis is confirmed preoperatively, the invaded RLN can be resected. Treatment options when functional deficits are expected after surgery include injection laryngoplasty, type 1 thyroplasty, arytenoid adduction, and immediate RLN reconstruction. Although vocal cord medialization procedures can be performed simultaneously with thyroid surgery, they can also be performed postoperatively after careful evaluation due to the difficulty in determining the function of the vocal cord during surgery [83]. As vocal cord hemorrhage, edema, and contralateral vocal cord palsy can lead to airway obstruction, patients are usually observed for several months after surgery; phonosurgery is reserved for patients who desire improvement of their voice. Immediate RLN reconstruction during surgery helps restore maximum function by regenerating the nerves and muscle. The best reinnervation results will likely be achieved if the new axons reach the target muscles rapidly, as this will enable them to recover from muscle atrophy and allow tension restoration [84,85]. Several reinnervation techniques have been reported, including direct anastomosis, free nerve grafting (e.g., using the great auricular nerve, vagus nerve, or ansa cervicalis), and creation of a laryngeal nerve-muscle pedicle [86]. Yumoto et al. [85] reported a superior mean flow rate, jitter, and glottal gap in patients who had undergone immediate RLN reconstruction versus arytenoid adduction simultaneously with thyroid surgery.
C3. What is the most appropriate surgical procedure for DTC with laryngeal invasion?
Recommendation 13
(A) Complete resection of the invaded tissue with adequate margins should be performed for DTC with laryngeal framework invasion. (Conditional recommendation/low-quality evidence)
(B) If outer cortex invasion of thyroid or cricoid cartilage is suspected, shaving resection of the cartilage can be con-sidered. (Conditional recommendation/low-quality evidence)
(C) If the tumor has penetrated the thyroid or cricoid carti-lage, partial or total resection of the larynx can be con-sidered. (Conditional recommendation/low-quality evidence)
Although DTC infiltration into the larynx is rare (1%–13%), infiltration into the laryngeal lumen can cause airway obstruction, asphyxia, and hemorrhage, which lead to death, so complete resection of the invaded tissue is essential [87]. Patients usually have symptoms, such as hoarseness, hemoptysis, and shortness of breath. A preoperative radiological evaluation by US and/or CT and/or MRI is required. Mucosal discoloration, thickening, and intraluminal invasion of the larynx and pyriform sinus should be checked before surgery using laryngoscopy, and in some cases, an intraoperative endoscopic evaluation is required [8].
Surgery should be planned taking into account the extent of disease, the patient’s general condition and surgical morbidity, the patient’s needs, and the surgeon’s experience and ability [88-92]. The extent of surgical resection differs somewhat depending on whether the tumor has invaded through the laryngeal cartilage into the lumen or shows only superficial extraluminal invasion. In the latter case with only superficial extraluminal invasion, partial thickness excision of laryngeal cartilage or shaving excision may be preferred over organ-sacrificing procedures, as a wide safety margin is not essential considering the less aggressive nature of DTC [93]. However, if the tumor has invaded the lumen of the larynx, complete resection of the invaded tumor with partial or total resection of the larynx may be considered. More conservative surgery with adjuvant therapy (radioactive iodine or radiation therapy) may be another option, despite the risk of leaving microscopic disease or a positive margin, to avoid resection of the larynx. However, total resection of the larynx may be necessary for salvage surgery because of the high failure rate of local control and the short time to recurrence [83,94]. No significant differences were found in the survival rates of patients undergoing shaving excision (conservative) versus complete excision, but the survival rate of patients with incomplete resection with gross residual disease was significantly reduced compared with those treated with conservative and complete excision. Two clinical studies that divided the patients into conservative, complete, and incomplete excision subgroups reported 5-year disease-specific survival rates of >85%, >85%, and 50%, respectively (n =124), and 92.3%, 83.9%, and 44.4%, respectively (n=64) [38,93,95,96].
D. Surgery for invasion of the trachea
D1. What is the most appropriate surgical procedure for DTC with tracheal invasion?
Recommendation 14
(A) If tracheal invasion of DTC is suspected, complete resec-tion of the invaded tissue with adequate margins should be performed. (Strong recommendation/moderate-quality evidence)
(B) Without endoluminal invasion of the trachea, shaving partial resection of the trachea can be considered. (Conditional recommendation/low-quality evidence)
(C) If endoluminal invasion of DTC is confirmed, window resection or sleeve resection should be considered. (Conditional recommendation/low-quality evidence)
Tracheal invasion by DTC is uncommon, occurring in approximately 3.4%–13% of cases [9]. Tracheal invasion adversely affects the long-term prognosis and survival, especially intraluminal invasion [95,97]. In addition, some reports have stated that the prognosis worsens with the degree of tracheal invasion [97,98]. However, there have been insufficient high-quality studies due to the rarity of such cases. Therefore, several guidelines, including the 2015 ATA management guidelines for adult patients with thyroid nodules and DTC, do not provide recommendations for the surgical management of tracheal invasion of DTC [17]. Failure to achieve clear margins according to the final pathological report has a significant adverse effect on survival [88,99-101]. Some studies have also reported no significant differences in the survival rate between microscopically negative and positive groups in cases with macroscopically negative margins [93,102]. Other studies reported no significant difference in the overall or disease-free survival rate between patients with a negative resection margin (R0) and those with a microscopic positive margin (R1) [103]. However, another study reported that the 5-year disease-specific survival rate decreased as the extent of resection became more limited, with rates of 94.4% for R0, 87.6% for R1, and 67.9% for gross disease (R2) [104]. A study of 103 DTC patients showed a significant difference in the 10-year survival rate between the R0 and R1 groups (65.5% vs. 27.3%, respectively) [105]. If the tumor can be completely resected with a wide margin, a 10-year survival rate >90% can be achieved [106].
There are three main methods of managing tracheal invasion in DTC patients [107,108]. Shaving partial resection is a method of gradually shaving the tracheal cartilage using a scalpel-like tool, while preserving the tracheal mucosa. Window resection is a method of excising all layers of the trachea, including the mucosa, in the tumor area with a safety margin; depending on the size of the defect after window resection, end-to-end anastomosis may be performed by performing primary closure, applying a muscular rotational flap, or performing a stepwise trachea split [99,109,110]. Sleeve resection of the trachea is a method involving removal of the entire ring and anastomosis of the top and bottom of the cut trachea [111]. There have been no reliable studies to determine which of these methods should be selected. Shin et al. [98] proposed a treatment method based on the classification of four patterns of tumor invasion of the tracheal cartilage [112]. Shaving partial resection can be a more effective surgical method if oncological safety is ensured, as it has a lower probability of postoperative complications than window resection and sleeve resection [103]. In cases of DTC with tracheal invasion, there have been reports that the recurrence rate is high following shaving partial resection because it is difficult to obtain an accurate negative margin due to subperichondrial invasion or invasion through the intercartilaginous ligament [88,100,113]. However, in those studies, the numbers of cases were small, and the surgical methods were not clearly distinguished according to the degree of tracheal invasion in many cases [114]. The shaving method proceeds macroscopically, confirming the tumor margin when superficial invasion of DTC into the tracheal cartilage has occurred [115]. A better prognosis can be expected if carcinoma-free results are obtained on frozen biopsy of the surrounding tissues [102]. Tsukahara et al. [110] reported that local control was possible in 21 of 22 patients (95%) with tracheal shaving when the tumor had not invaded the tracheal mucosa. In a study of 69 patients, Kim et al. [116] reported no significant difference in the local recurrence rate between patients who underwent shaving excision and those who underwent more extensive surgery, even when a microscopic positive margin was noted in the permanent pathological report. In addition, the disease-specific survival rate was reported to be 100% after 10 years of follow-up. In a meta-analysis, Warshavsky et al. [117] reported that, among 284 patients with tracheal invasion, there was no significant difference in the 10-year overall survival rate of patients who underwent shaving partial resection versus window or sleeve resection. In another meta-analysis, Svider et al. [108] reported that the locoregional recurrence rates after window resection and sleeve resection were 15% and 25.6%, respectively, and the overall survival rates were 77.1% and 74.5%, respectively. Evidence regarding the most appropriate indications for window resection remains insufficient. Moritani et al. [118] recommended performing window resection for tumors that have invaded up to half of the circumference of the tracheal wall, while other reports recommended sleeve resection when the tumor has invaded the tracheal mucosa because of the possibility of tumor spread to the opposite side [113,119]. Further large-scale prospective studies are required to determine which surgical method should be chosen according to the status of tracheal invasion.
E. Surgery for esophageal invasion
E1. What is the most appropriate surgical procedure for DTC with esophageal invasion?
Recommendation 15
(In cases where esophageal invasion of DTC is suspected, complete resection of the invaded tissue with adequate mar-gins should be performed during surgery.
(Conditional recommendation/low-quality evidence)
The rate of esophageal involvement of locally invasive DTC is reported to be 21%, which is relatively infrequent compared with involvement of the muscles (53%), RLN (47%), or the trachea (37%) [1]. Esophageal invasion commonly occurs together with tracheal invasion from posteriorly located cancers [2]. It can also occur from extranodal extension of paratracheal or paraesophageal lymph node metastasis.
Whereas the mucosa of the esophagus and pharynx is relatively resistant to direct invasion, the muscular layer may be readily invaded, leading to significant compressive dysphagia. Therefore, invasion is usually confined to the muscular layer, with preservation of the mucosa and submucosal layer. If esophageal invasion is suspected based on preoperative radiological evaluation, esophagoscopy or endoscopic US may provide additional information about the extent of disease invasion, aiding in surgical planning [3]. If the ETE involves only the external muscular layer, complete resection of the invaded muscular layer with preservation of the submucosa may be adequate. During resection, the tumor can be dissected from the underlying mucosa by developing a submucosal plane without difficulty. If tears in the underlying mucosa are created, primary repair is required to prevent salivary leakage.
If the tumor extensively involves all three layers, partial esophageal resection or circumferential esophagectomy should be performed with appropriate reconstruction, such as free tissue transfer, use of a pedicled myocutaneous flap, or gastric, colonic, or jejunal tissue transfer [120]. Recently, a surgical technique for simultaneous tracheal and esophageal reconstruction using a free bipaddled posterior tibial artery perforator was reported [121].
Complete resection of esophageal invasion with an adequate margin is considered the main goal of surgical treatment [90,94,120,122-126], and the prognosis of patients with incomplete resection (16%) was significantly poorer than that of patients with complete resection, including sharp dissection (80%) or total resection (100%) [123]. Tanaka et al. [124] reported 15-year survival rates of 100% and 74.2% in the complete and incomplete resection groups, respectively, among patients with locally invasive PTC. Hartl et al. [126] reported a trend for poorer local control in patients with R1 versus R0 margins, and a higher rate of R0 resection was obtained with full-thickness resection.
Segal et al. [13] reported that the type of surgery (radical vs. conservative) did not have a significant effect on survival in thyroid cancer patients with involvement of the esophagus. For inoperable tumors, gastrostomy tubes and esophageal stents may be considered as palliative treatment.
F. Surgery for patients exhibiting central and lateral cervical lymph node involvement
F1. Is prophylactic neck dissection necessary for patients with clinically N0 locally invasive DTC?
Recommendation 16
Prophylactic central compartment neck dissection should be considered in patients with clinically node-negative locally invasive DTC.
(Conditional recommendation/moderate-quality evidence)
Neck management is one of the most important components of the treatment of advanced PTC. Many studies have investigated the treatment outcomes of the routine use of prophylactic central neck dissection (PCND) for PTC. However, most were retrospective, single-center, studies and yielded contradictory results, and the study participants generally included patients with all disease stages or were limited to patients with early-stage disease, such as papillary microcarcinoma. A systematic search found no studies specifically focusing on the role of PCND in patients with locally advanced DTC (T3 or T4N0). Some studies did not include control participants. Therefore, in-depth subgroup analyses were performed, although the quality of evidence was inevitably low.
To evaluate disease recurrence and survival, a relatively long-term follow-up period is required because PTC has indolent tumor characteristics. In a large-scale retrospective analysis of 4,371 patients, Ito et al. [127] found excellent 10- and 20-year recurrence-free survival rates after PCND. In detailed analyses, significant ETE, an indicator of T3 stage, and a tumor size >4 cm were associated with significantly decreased recurrence-free survival, indicating the need to consider PCND in high-risk patients [127]. However, that was a single-arm study without a control group. Studies have suggested that PCND is beneficial for PTC cases with a high risk of lymph node metastasis and large (>1–4 cm) tumors [128,129]. From this perspective, some researchers have argued in favor of bilateral PCND, as the rates of permanent hypoparathyroidism or RLN injury are not different between total thyroidectomy with PCND and total thyroidectomy without PCND, but the survival rate is increased: the 10-year disease-specific survival rate of patients who underwent total thyroidectomy without CND was 92.5% compared to 98.0% in patients with CND (P =0.034). Multivariate analysis showed that the presence of ETE was an independent factor predicting locoregional recurrence (odds ratio [OR], 12.47; 95% confidence interval [CI], 6.74–23.06), and that PNCD was beneficial for such cases (OR, 0.21; 95% CI, 0.11–0.41) [130]. The recent guidelines of the European Society for Medical Oncology cited these results and suggested using PCND to improve regional control in invasive PTC [131]. Consistent with these observations, a subgroup analysis based on ATA risk stratification showed significantly lower recurrence rates after PCND in intermediate- and high-risk groups, while there was no significant difference in the low-risk group [132]. Another group also argued for bilateral PCND, as permanent hypoparathyroidism and unintentional recurrent nerve paralysis occurred infrequently (2/317 cases each), and bilateral PCND optimized the staging, providing a basis for a personalized approach to adjuvant radioiodine treatment: patients with stage pN0 received less radioiodine than patients with stage pN1 (median: 30 vs. 100 mCi, respectively, P<0.0001) [133]. Others suggested that unilateral PCND is sufficient, as metastasis to the contralateral paratracheal lymph node had a low incidence of 4.2% in the bilateral PCND group [134].
There have been conflicting reports regarding recurrence-free survival after PCND [135,136]. One study suggested that PCND did not significantly decrease the risk of locoregional recurrence, but significantly increased the rates of temporary vocal cord palsy (5.6% vs. 2.5%, respectively; P =0.001), temporary hypoparathyroidism (30.8% vs. 16.7%, respectively; P <0.001), and permanent hypoparathyroidism (3.5% vs. 1.7%, respectively; P <0.001) compared with no PCND [136]. In a meta-analysis, the overall recurrence rate was 2.02% with PCND versus 3.92% without PCND (OR, 1.05; 95% CI, 0.48–2.31) [137]. The recurrence rate was 1.86% versus 1.68% in the central neck compartment (OR, 1.31; 95% CI, 0.44–3.91) and 3.73% versus 3.79% in the lateral neck compartment (OR, 1.21; 95% CI, 0.52–2.75) in the presence versus absence of PCND, respectively, and these differences were not statistically significant [137]. However, the meta-analysis was not performed exclusively in patients with advanced disease and therefore requires caution in interpretation of the results. A recently published randomized controlled trial showed no differences in oncologic outcomes at 1 year after surgery or in the complication rate in the presence versus absence of PCND; however, the inclusion criteria were not limited to advanced disease, the follow-up period was short, and the number of patients was small [138].
We could not find sufficient evidence for the usefulness of lateral neck dissection with a fair evaluation of the risks and benefits of PLND. Only one study suggested the application of PLND in cases with a primary tumor >2 cm because the lateral neck nodes are positive in cases of large primary tumors [139]. Considering the significant risk of complications associated with lateral neck dissection, the panel cannot recommend for or against PLND for clinically N0 locally advanced PTC (cT3 or T4), as there is no evidence that this procedure leads to reduced recurrence or improved survival.
F2. What extent of neck dissection is appropriate for DTC patients with clinically positive neck nodes?
Recommendation 17
If preoperative clinically evident central neck node metastasis is identified, ipsilateral central compartment neck dissection (level VI) is recommended.
(Strong recommendation/moderate-quality evidence)
Although some authors have reported that PTC lymph node metastasis has no clinically important effect on the outcomes of low-risk patients, a study of the SEER database found that among 9,904 patients with PTC, lymph node metastasis, age >45 years, distant metastasis, and large tumor size significantly predicted poor overall survival on multivariate analysis [140]. Another SEER registry study concluded that cervical lymph node metastasis conferred an independent risk of decreased survival, but only in patients with follicular cancer and patients with papillary cancer over age 45 years [141]. A recent comprehensive analysis of the National Cancer Database and SEER, however, showed a small but significant increase in mortality risk for patients younger than 45 years with lymph node metastasis compared with younger patients without lymph node involvement, and demonstrated that having incrementally more metastatic lymph nodes with up to six involved nodes conferred an additional mortality risk in this age group [143].
Although it is difficult to accurately detect lymph node metastasis before surgery, preoperative examination using US is the most sensitive method, with specificities in the central, ipsilateral, and contralateral compartments of 95%, 93%, and 96%, respectively [144]. The sensitivity and specificity of US and Doppler US patterns for identifying abnormal lymph nodes are summarized in Table 2 [145-149].
The locations of the involved lymph nodes are of particular importance when attempting to identify malignancy. Almost half of all metastatic lymph nodes are located at levels III and IV, with the other half at level VI [147]. Based on the data shown in Table 2, cervical lymph nodes can be classified into three groups:
Normal:Hilum preserved, ovoid shape and normal size, absent or hilar vascularization, no other suspicious signs.
Indeterminate:Absence of a hilum and at least one of the fol-lowing characteristics: round shape, increased short axis ≥8 mm at level II and ≥5 mm at levels III and IV, increased central vascularization.
Suspicious for malignancy:At least one of the following char-acteristics: microcalcifications, partially cystic appearance, pe-ripheral or diffusely increased vascularization, hyperechoic tissue with a thyroid-like appearance.
The role of therapeutic lymph node dissection for the treatment of thyroid cancer nodal metastasis is well accepted for cN1 disease [140,150,151]. Focal “berry picking” of only clinically involved lymph nodes without compartmental dissection leads to higher rates of recurrence and should be abandoned. Compartment central neck dissection is recommended for clinically central neck-positive patients, and this therapeutic neck dissection involves removal of the pretracheal and prelaryngeal lymph-bearing tissue, along with unilateral paratracheal nodal removal. Musacchio et al. [152] reported that a limited “berry picking” operation in neck node-positive patients showed a recurrence rate of 100%, which was significantly poorer than that for formal central neck dissection (9%). Noguchi et al. [153] reported similar results, with an overall recurrence rate of 17.4% in patients who underwent “node picking” operations versus 9.1% in patients who underwent compartment neck dissection.
Generally, in clinically central neck-positive patients, the main principle is to perform ipsilateral compartment central neck dissection. However, some authors have different perspectives regarding the extent of surgery. Keum et al. [154] recommended bilateral central neck dissection when therapeutic lateral neck dissection is performed due to the high rate of occult metastasis (27.0%) and the low sensitivity of imaging studies in the contralateral central compartment (19.0%). Although the relationships between BRAF mutation and the clinicopathological characteristics of papillary thyroid cancer have been investigated, the role of BRAF mutation in determining the extent of neck dissection remains unclear [155]. Bae et al. [156] emphasized the significance of right paraesophageal lymph node dissection because of the high rate of metastasis in this node (19.6%) in clinically central neck-positive patients.
Recommendation 18
If lateral cervical lymph node metastasis is confirmed by biopsy, therapeutic compartment neck dissection is recommended.
(Strong recommendation/moderate-quality evidence)
For patients with clinically evident lateral cervical lymph node metastasis on preoperative US-guided FNA cytology or washout Tg measurement or frozen biopsy at the time of surgery, the risks of recurrence and mortality may be reduced by surgical resection [141,143,157,158]. Ito et al. [158] showed that the presence of clinically apparent lymph node metastasis in the lateral compartment detected on US is useful without FNA cytology. One series showed an insufficiently determined surgical extent in the absence of preoperative FNA or intraoperative frozen-section evaluation [157].
As it has been recommended to avoid “berry picking” because of its association with a higher rate of recurrence [152] and the risk of missing metastasized lymph nodes [159], surgical resection based on compartmental lymph node dissection could be regarded as the standard procedure. To determine the extent of comprehensive compartmental cervical lymph node dissection, it is necessary to confirm the pattern of the metastasis. Thyroid cancer often metastasizes to the level II–V compartments, and involvement of level I has also been reported [160]. Among the compartments, level III is the most common site of lateral cervical neck metastasis [160]. However, debate continues regarding the neck levels to be removed for well-differentiated thyroid carcinoma, with several authors drawing different conclusions. For example, level II is widely included in the extent of comprehensive neck dissection [161-163], but it was not proven to be necessary to remove level IIb (a subsection of level II) without evidence of metastasis in level IIb [162,163]. There is also debate about level V resection in cN1b patients [154,164]. However, most studies have agreed on the necessity of removing levels II, III, and IV based on the distribution of lateral cervical neck metastasis [154,160,161,164-170].
Recently, contradictory results were reported for patients with lateral cervical neck metastasis without clinically evident central neck metastasis. Harries et al. [168] reported no significant difference in the central neck recurrence-free survival rate in their patients with N1b who underwent lateral neck dissection, but not PCND, compared with patients who underwent lateral neck dissection combined with PCND. However, numerous studies support lateral neck dissection and PNCD for patients with cN1b [154,165,166,169]. In this respect, neck dissection of levels II, III, IV, and VI should generally be performed in cases of lateral cervical neck metastasis without involvement of level I or V.
F3. What is the most appropriate extent of neck dissection for patients with recurrent/persistent neck node involvement in DTC?
Recommendation 19
(A) If a compartment in which lymph nodes have not been dis-sected previously exhibits disease recurrence, nodal clear-ance in a compartmental fashion should be performed. (Conditional recommendation/low-quality evidence)
(B) If a compartment in which lymph nodes have been dissected previously exhibits disease recurrence, the extent of nodal clearance should be decided after considering the oncolog-ical benefit of compartment nodal clearance and the poten-tial risk of complications associated with revision surgery. (Conditional recommendation/low-quality evidence)
Compartment-oriented dissection should be performed to eradicate the likely involvement of multiple nodes and to reduce the risk of having to return to that compartment to remove additional nodes. ‘‘Berry picking’’ of the affected lymph nodes should be avoided. The goal of revision surgery should be to clear the involved nodal basins in a compartmental fashion and to dissect nodal basins likely to harbor microscopic regional metastases [170]. However, if the compartment in which a lymph node has recurred has been dissected previously, repeated dissection of the entire compartment may be risky or difficult. Therefore, a more targeted approach to a particular lesion depending on the scarring encountered when removing the recurrent node may be preferred [171].
For level VI recurrence, it is especially important to reduce complications, such as hypoparathyroidism and RLN injury. Therefore, the American Head and Neck Society Consensus Statement suggested that it is preferable to avoid bilateral central compartment dissection in the absence of bilateral disease. Even in cases with bilateral level VI recurrence, contralateral central compartment dissection may be staged [170]. Charcoal tattooing under US guidance is an easy, safe, and useful procedure for surgical guidance in neck reoperation for thyroid cancer. Chami et al. [172] reported charcoal tattooing in a total of 106 lesions, and the tolerance of charcoal injection was good in all but three patients. A mean volume of 1 mL of charcoal was injected with a mean of two targets per patient. Charcoal labeling facilitated intraoperative detection in 56 “difficult” lesions and facilitated intraoperative guidance in 17 lesions. The feasibility and usefulness rates were 83% and 70.7%, respectively. A study in Hong Kong even suggested that the recommended threshold (8 mm) for the largest node size may be too stringent an indication for revisional CND and could be raised to 15 mm without increasing the surgical morbidity from revision central neck dissection [173]. In contrast, Clayman et al. [174] reported that bilateral comprehensive level VI/VII dissection is safe and effective for recurrent/persistent PTC in the central compartment. They reported a permanent hypoparathyroidism rate of 1% and recurrent nerve palsy rate of 2%. The postoperative recurrence rate in the central compartment was 10% (median interval, 24.3 months) after the first operation. The postoperative central compartment recurrence rate was 2% after the second operation. The disease-specific survival rates at 10 years were 98.9% for patients <45 years and 77.9% for patients ≥45 years [174]. Another study suggested that compartment dissection is preferred over a targeted approach. Risk factors for incomplete response after reoperation were age ≥45 years, aggressive histology, and lymph node ratio ≥0.6 at initial surgery. Risk factors for secondary relapse following complete remission achieved with reoperation were male sex, aggressive histology, and ≥10 metastases [175].
Compared with central compartment reoperation, some studies have emphasized the need for compartment-oriented dissection rather than a targeted approach in lateral compartment reoperation. Chinn et al. [176] showed that comprehensive lateral neck dissection of level II-V achieved a very high in-field regional control rate and ultimate lateral neck control rate in recurrent/ persistent DTC. In their study, recurrence occurred most commonly at levels III and IV (33% and 33%, respectively). The infield lateral neck control rate was 96% at 10 years, and the overall lateral neck regional control rate was 88% at 10 years. The 10-year overall and disease-specific survival rates were 78% and 91%, respectively. Those authors recommended comprehensive formal lateral neck dissection from level II to V by experts, with rare perioperative complications [176]. In addition, a study at Johns Hopkins Hospital showed that lateral neck recurrence after total thyroidectomy with radioactive iodine therapy is likely to appear as multiple lymph nodes at multiple neck levels, with rates of multiple-level lymph node involvement of 77% and 64% after the first and second lateral neck dissection, respectively [177].
CONCLUDING REMARKS
The evidence-based guidelines developed in this study, on the basis of a systematic review of clinical reports, are intended to help clinicians make safer, more efficient clinical decisions for the optimal surgical treatment of patients with locally invasive DTC. There may be differing views, and the best choice for each patient may vary according to the patient’s condition or medical environment. Although it is hoped that these guidelines will be useful in clinical practice, they do not constitute regulations and do not have any legal force. The responsibility for treatment outcomes in actual clinical practice rests directly with clinicians in charge of treatment.
HIGHLIGHTS
▪ Although the prognosis of differentiated thyroid cancer (DTC) is generally good, locally invasive DTC with gross extrathyroidal extension may lead to several functional deficits and poor oncological outcomes.
▪ The primary goal of surgery is the complete eradication of all cancer tissues with a safe margin; however, this is not always achievable because radical resection is challenging and carries a risk of complications.
▪ At present, the optimal extent of surgery remains a matter of debate; therefore, we developed these guidelines to help surgeons make clinical decisions for patients with locally invasive DTC.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: JOP, JHK, YHJ, EJC, YBJ, KHO, HSL, SKB, SYK. Data curation: all authors. Formal analysis: JOP, JHK, SKB. Methodology: all authors. Project administration: SKB, SYK. Visualization: JOP, JHK. Writing–original draft: JOP, JHK. Writing–review & editing: JOP, JHK, SKB, SYK.
Supplementary materials
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2022.01732.
The search string used for each key questions
Flow diagram of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
Quality of literature and evidence level, and grades of recommendations