|
 |
- Search
AbstractObjectivesWe wanted to evaluate whether the presence of nasal obstruction makes a change on the association between the modified Mallampati score and the severity of sleep-disordered breathing (SDB) and the sleep quality.
MethodsPolysomnography (PSG), the modified Mallampati score (MMS), the body-mass index, and a questionnaire about nasal obstruction were acquired from 275 suspected SDB patients. The subjects were divided into two groups according to the presence of nasal obstruction. The clinical differences between the two groups were evaluated and the associations between the MMS and PSG variables in each group were also assessed.
ResultsSignificant correlations were found between the MMS and many PSG variables, including the apnea-hypopnea index, the arousal index and the proportion of deep sleep, for the patients with nasal obstruction, although this was not valid for the total patients or the patients without nasal obstruction.
Sleep-disordered breathing (SDB) is characterized by periodic reductions or cessation of the airflow during sleep and this leads to hypoventilation, apnea and sleep fragmentation. SDB is mostly caused by narrowing of the upper airway tract. Collapsibility of the upper airways is one of the mechanisms involved in the pathogenesis of SDB, and a large tongue base could be associated with a higher risk of SDB because it can make the airway narrower [1].
Nasal breathing is the preferred route of breathing when we are awake and sleep. The relationship between the nasal airway and the collapse of the upper airways is complex, and the precise role played by the nasal airway in SDB is as yet unknown. But if a given patients has nasal obstruction, then they must breathe through the oral cavity and this mouth breathing lengthens and narrows the upper airway and makes it more collapsible to inspiratory negative pressure [2]. It may cause breathing through the upper airway more difficult to perform and sleep becomes more fragmented.
So in this study, the authors tried to evaluate whether the presence of nasal obstruction makes a change on the association between the modified Mallampati score (MMS) and the severity of SDB and sleep quality.
We enrolled the consecutive adult patients who were more than 18 years old with suspected SDB and who underwent polysomnography (PSG) from January 2009 to May 2010. The study was approved by the institutional ethics committee. They had been referred to our department with complaints of snoring and sleep apnea. The patients we excluded from this study had already undergone an operation for obstructive sleep apnea syndrome (OSAS) or they had shown evidence of reduced cardiac function and/or chronic obstructive pulmonary disease at the time of the study. The patients with incomplete data were also excluded. The patients were interviewed and given a questionnaire on whether they usually have nasal obstruction when they are in the supine position and the patients were assigned to the 'without-nasal-obstruction' group and the 'with-nasal-obstruction' group.
All the patients received a full otolaryngologic evaluation, including the modified MMS, and they had undergone diagnostic PSG. The MMS is often used to assess the tongue base and its relation to the soft palate [3]. All the subjects had their height and body weight recorded and the body-mass index (BMI) was calculated. The MMS was evaluated according to the Friedman's classification described in our earlier study [4,5].
PSG was performed using the Alice3 (Healthdyne Technologies, Marietta, GA, USA) or Somnologica system (Embla, Broomfield, CO, USA). The day before the sleep studies, subjects were asked not to drink alcohol or caffeinated beverages. Overnight polysomnography was performed using a 4-channel electroencephalogram (EEG; C3/A2, C4/A1, O1/A2, O2/A1), a 4-channel electrooculogram, an electromyogram (the submental, intercostal and anterior tibialis muscles, and an electrocardiogram with surface electrodes. A thermistor (for monitoring nasal airflow), a nasal air pressure monitor, an oximeter (for measuring oxygen saturation), piezoelectric bands (for determining thoracic and abdominal wall motion), and a body position sensor were also attached to the patients. The subjects were recorded on videotape using an infrared video camera and they were continuously observed by a PSG technician. The sleep architecture was scored in 30 second, epochs, and the sleep staging was interpreted according to the standard criteria of Rechtschaffen and Kales.
Apneas and hypopneas were defined by previously reported criteria [6]. Obstructive apnea was defined as a reduction in the airflow >90% lasting Ōēź10 seconds during which time there was evidence of persistent respiratory effort. Hypopnea was defined as reduction in airflow by 50% with a duration Ōēź10 seconds or a reduction of airflow by 30% for more than 10, and this was accompanied by EEG arousal and/or 3% or greater oxygen desaturation.
According to the American Sleep Disorders Association Task Force criteria [6], arousals were classified as breathing-related arousals (occurring within 3 seconds following apnea, hypopnea or snoring) and other type of arousals (spontaneous arousal, periodic limb movement-associated arousals). The percentage of time spent in the non-rapid eye movement state versus the time spent in the rapid eye movement (REM) state and the central apnea were also recorded.
Statistical analysis was performed with SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Spearman correlation analysis was performed to evaluate the associations between the MMS and PSG variables, including apnea-hypopnea index (AHI), arousal index and the proportion of deep sleep (deep sleep%) and REM sleep (REM sleep%). Partial Spearman correlation analysis was performed with adjustment for possible covariates. This statistical evaluation was also separately performed in the patient groups with and without nasal obstruction.
Chi-square tests were performed to verify the differences of gender, the SDB severity and the MMS according to the presence of nasal obstruction. Student t-test was used to evaluate the difference of BMI and age between the patients with and without nasal obstruction. The significance level was set at P<0.05 for all the analyses.
Two hundred ninety eight patients visited our clinic and underwent PSG during the recruitment period. Twenty three patients were excluded from the study because of incomplete PSG data (n=9), data loss about nasal obstruction (n=8), known bronchial asthma (n=2), and previous OSAS surgery (n=4).
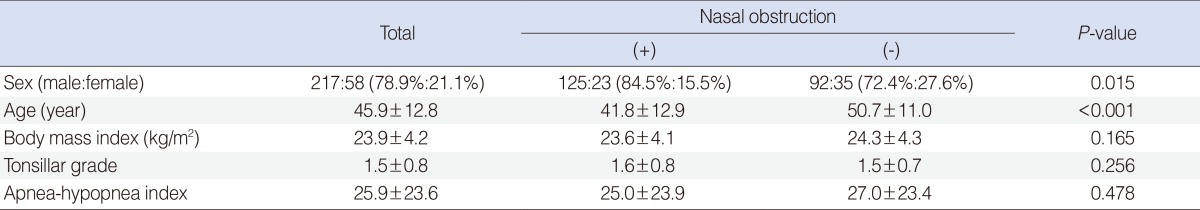
So, 275 patients were finally included in this study. They were composed of 217 males and 58 females. The female to male ratio was significantly higher in the without-nasal-obstruction group (P=0.015). The mean age was 45.9┬▒12.8 and this was higher in the without-nasal-obstruction group (P<0.001). The mean BMI was 23.9┬▒4.2 and it showed no statistical difference between the two groups (P=0.165). Mean tonsillar grades didn't show any statistical difference between the two groups either (P=0.256). All the anthropometric data is summarized in Table 1.
The mean AHI was 25.9┬▒23.6 and the subjects were composed of 55 simple snorers (20.0%), 74 mild OSAS patients (26.9%), 48 moderate OSAS patients (17.5%), and 98 severe OSAS patients (35.6%) (Fig. 1). There was no statistical difference of the AHI between the two groups with/without nasal obstruction (P=0.478) (Table 1). The distribution of the MMS in the total patients was as follows: 23 patients (8.4%) were MMS I, 79 patients (28.7%) were MMS II, 70 patients (25.5%) were MMS III and 103 patients (37.5%) were MMS IV. The distribution of the MMS showed no statistical difference between the patients with and without nasal obstruction (P=0.178) (Fig. 2).
No significant correlation was found between the MMS and the PSG parameters such as total AHI, arousal index, REM sleep% (REM sleep time/total sleep time├Ś100), the deep sleep% (deep sleep time/total sleep time├Ś100) and the lowest SaO2 for the total subjects, although there was some tendency for a correlation between the MMS and the total AHI (P=0.058). Analysis using partial Spearman correlation controlling for age, sex, tonsillar grade, and presence of nasal obstruction also revealed similar results (Table 2).
However, significant correlations were found between many of the PSG variables and the MMS when the subjects were divided according to the presence of nasal obstruction. In the patient group with nasal obstruction, a significant association was found between the MMS and sleep disordered breathing. The total AHI and arousal index became higher as the MMS was increased (Table 2). Sleep quality was also influenced by the MMS in the patients with nasal obstruction. Deep sleep showed a significant decrease according to the increase of the MMS. The lowest SaO2 showed a decreasing tendency as the MMS increased even though it didn't reach statistical significance. Analysis using partial Spearman correlation controlling for age, sex, and tonsillar grade also revealed similar results (Table 2).
There was little significant correlation found for the patients without nasal obstruction. Only the REM sleep% showed significant correlation with the MMS (Table 2). However, after controlling for possible covariates including age, sex, and tonsillar grade, the REM sleep% didn't showe significant correlation with the MMS any more.
The present study showed that the MMS might be associated with SDB and the sleep quality in patients with nasal obstruction. An elevated AHI and arousal index and a decreased % of deep sleep were noted in the patients with higher MMS and nasal obstruction even though the association is low (Spearman's Žü for AHI=0.210). These results were confirmed with partial Spreaman correlation analysis controlling for possible covariates.
Nasal breathing is the preferred route of breathing when we are awake and sleep, and the nasal airway is responsible for approximately two third of the total airway resistance in wakefulness [7]. The contribution of nasal obstruction to SDB has been investigated in many articles. Complete nasal packing can cause poorer oxygen saturation in most healthy subjects and it can cause pathologic desaturation (an oxygen desaturation indexŌēź12) in about half of the subjects [8]. However, the relation between nasal patency and the sleep parameters is very weak in real SDB patients and there is a dispute over the association between the increase of nasal resistance and SDB, although there have been some reported contradictory results [9-12]. Medical and surgical treatments for nasal obstruction also have various results for SDB, although some studies have reported that it improves sleep quality [13-18].
Yet there is an interesting report about the SDB patient subgroups. Series et al. [19] reported that SDB patients with normal cephalometric variables, including the mandibular plane-hyoid length, had better postoperative PSG parameters after surgery. Mouth breathing that may be caused by nasal obstruction lengthens and narrows the upper airway and makes it more collapsible to inspiratory negative pressure [2]. From this result, we can assume that the presence of nasal obstruction may aggravate retroglossal narrowing during sleep. That's why we started the present study.
The present study showed that the SDB patient subgroup with nasal obstruction might be more influenced by a high MMS, which represents the size of airway in the tongue base level. There are two possible hypotheses for this association between nasal obstruction and MMS.
First, nasal obstruction can aggravate mouth breathing because impaired nasal breathing precipitates mouth opening during respiration when nasal resistance exceeds a certain level. This switch from nasal to oral breathing is disadvantageous physiologically and mouth breathing is associated with a reduction of the retropalatal and retroglossal areas and it lengthens the pharyngeal airway as a result of further posterior retraction of the tongue, which might result in elevation of AHI during sleep [1,2]. Although the upper airway resistance is similar between the oral and nasal breathing routes during wakefulness, it is much higher while breathing orally than when breathing nasally during sleep [20]. A higher modified Mallampati score might have a more chance of resulting in the elevation of AHI during sleep with mouth breathing. Treatment of severe nasal obstruction can reduce mouth breathing during sleep [21,22].
Second, the nasal airway has a more rigid frame and the pharyngeal airway is surrounded by soft tissue, including the tongue and pharyngeal wall acting like Starling's resistor model [23]. Elevated nasal resistance upstream results in increased negative pressure (suction force) in the oropharyngeal airway downstream [24]. So, a larger tongue base and a narrower pharyngeal airway can be more influenced by the increased negative pressure caused by nasal obstruction [25].
In the present study, we used the subjective sense of nasal obstruction as the standard for dividing the groups. There is little evidence whether objective methods for measuring nasal resistance (rhinomanometry) or cross-sectional areas (acoustic rhinometry) are better as a classification standard for nasal obstruction than a subjective method [26,27]. Although the objective methods have been frequently used to evaluate the relationship between SDB and the nasal obstruction caused by septal deviation, the available literature provides inconsistent evidence to support this relationship [24]. Yet in patients with allergic rhinitis, a direct association between nasal resistance and the SDB severity has been found as well as between nasal obstruction and the subjective quality of sleep and daytime sleepiness [28,29]. The severity of this variable nasal obstruction cannot be measured well with the objective methods, although epidemiological studies have shown that allergic rhinitis affects 9%-42% of the general population [24]. Therefore, We think the subjective method might have merits for evaluating nasal obstruction in these patients.
The present study showed the MMS is well correlated with the severity of SDB as well as the sleep quality, especially in patients with nasal obstruction. The MMS can give more valuable information about the severity of SDB to the sleep physician when combined with simple questions about nasal obstruction.
ACKNOWLEDGMENTSWe are grateful to Sook-young Woo in Biostatistics team for her expert consultation with statistical analysis.
References1. Kim HY, Bok KH, Dhong HJ, Chung SK. The correlation between pharyngeal narrowing and the severity of sleep-disordered breathing. Otolaryngol Head Neck Surg. 2008 3;138(3):289-293. PMID: 18312873.
2. Lee SH, Choi JH, Shin C, Lee HM, Kwon SY, Lee SH. How does open-mouth breathing influence upper airway anatomy? Laryngoscope. 2007 6;117(6):1102-1106. PMID: 17464234.
3. Friedman M, Tanyeri H, La Rosa M, Landsberg R, Vaidyanathan K, Pieri S, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999 12;109(12):1901-1907. PMID: 10591345.
4. Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope. 2004 3;114(3):454-459. PMID: 15091218.
5. Kim HY, Min JY, Cho DY, Chung SK, Dhong HJ. Influence of upper airway narrowing on the effective continuous positive airway pressure level. Laryngoscope. 2007 1;117(1):82-85. PMID: 17202935.
6. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992 4;15(2):173-184. PMID: 11032543.
7. Ferris BG Jr, Mead J, Opie LH. Partitioning of respiratory flow resistance in man. J Appl Physiol. 1964 7;19:653-658. PMID: 14195575.
8. Armengot M, Hern├Īndez R, Miguel P, Navarro R, Basterra J. Effect of total nasal obstruction on nocturnal oxygen saturation. Am J Rhinol. 2008;May-Jun;22(3):325-328. PMID: 18588768.
9. Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope. 1992 10;102(10):1150-1152. PMID: 1405965.
10. Miljeteig H, Savard P, Mateika S, Cole P, Haight JS, Hoffstein V. Snoring and nasal resistance during sleep. Laryngoscope. 1993 8;103(8):918-923. PMID: 8361295.
11. De Vito A, Berrettini S, Carabelli A, Sellari-Franceschini S, Bonanni E, Gori S, et al. The importance of nasal resistance in obstructive sleep apnea syndrome: a study with positional rhinomanometry. Sleep Breath. 2001 1;5(1):3-11. PMID: 11868135.
12. Lofaso F, Coste A, d'Ortho MP, Zerah-Lancner F, Delclaux C, Goldenberg F, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000 10;16(4):639-643. PMID: 11106205.
13. Craig TJ, Mende C, Hughes K, Kakumanu S, Lehman EB, Chinchilli V. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc. 2003;Jan-Feb;24(1):53-58. PMID: 12635578.
14. Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol. 1998 5;101(5):633-637. PMID: 9600500.
15. Hughes K, Glass C, Ripchinski M, Gurevich F, Weaver TE, Lehman E, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy. 2003 5;58(5):380-385. PMID: 12797340.
16. Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax. 2004 1;59(1):50-55. PMID: 14694248.
17. Rombaux P, Liistro G, Hamoir M, Bertrand B, Aubert G, Verses T, et al. Nasal obstruction and its impact on sleep-related breathing disorders. Rhinology. 2005 12;43(4):242-250. PMID: 16405266.
18. Vural C, Gungor A. The effect of topical fluticasone on nasal nitric oxide levels in a patient with allergic rhinitis. Ear Nose Throat J. 2003 8;82(8):592-597. PMID: 14503095.
19. Series F, St Pierre S, Carrier G. Surgical correction of nasal obstruction in the treatment of mild sleep apnoea: importance of cephalometry in predicting outcome. Thorax. 1993 4;48(4):360-363. PMID: 8511733.
20. Fitzpatrick MF, McLean H, Urton AM, Tan A, O'Donnell D, Driver HS. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003 11;22(5):827-832. PMID: 14621092.
21. Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008 1;31(1):110-117. PMID: 17898015.
22. McLean HA, Urton AM, Driver HS, Tan AK, Day AG, Munt PW, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005 3;25(3):521-527. PMID: 15738298.
23. Park SS. Flow-regulatory function of upper airway in health and disease: a unified pathogenetic view of sleep-disordered breathing. Lung. 1993 11;171(6):311-333. PMID: 8295427.
24. Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol. 2011 9;268(9):1365-1373. PMID: 21340561.
25. Mirza N, Lanza DC. The nasal airway and obstructed breathing during sleep. Otolaryngol Clin North Am. 1999 4;32(2):243-262. PMID: 10385535.
26. Andre RF, Vuyk HD, Ahmed A, Graamans K, Nolst Trenite GJ. Correlation between subjective and objective evaluation of the nasal airway: a systematic review of the highest level of evidence. Clin Otolaryngol. 2009 12;34(6):518-525. PMID: 20070760.
27. Kim CS, Moon BK, Jung DH, Min YG. Correlation between nasal obstruction symptoms and objective parameters of acoustic rhinometry and rhinomanometry. Auris Nasus Larynx. 1998 1;25(1):45-48. PMID: 9512794.
28. McNicholas WT. The nose and OSA: variable nasal obstruction may be more important in pathophysiology than fixed obstruction. Eur Respir J. 2008 7;32(1):3-8. PMID: 18591332.
29. McNicholas WT, Tarlo S, Cole P, Zamel N, Rutherford R, Griffin D, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982 10;126(4):625-628. PMID: 7125355.
Fig.┬Ā1Distribution of the sleep-disordered breathing severity in all the patients and the patients with or without nasal obstruction. OSAS, obstructive sleep apnea syndrome. 
Fig.┬Ā2Distribution of the modified Mallampati score (MMS) in all the patients and the patients with or without nasal obstruction. 
Table┬Ā2Polysomnographic data of the subjects 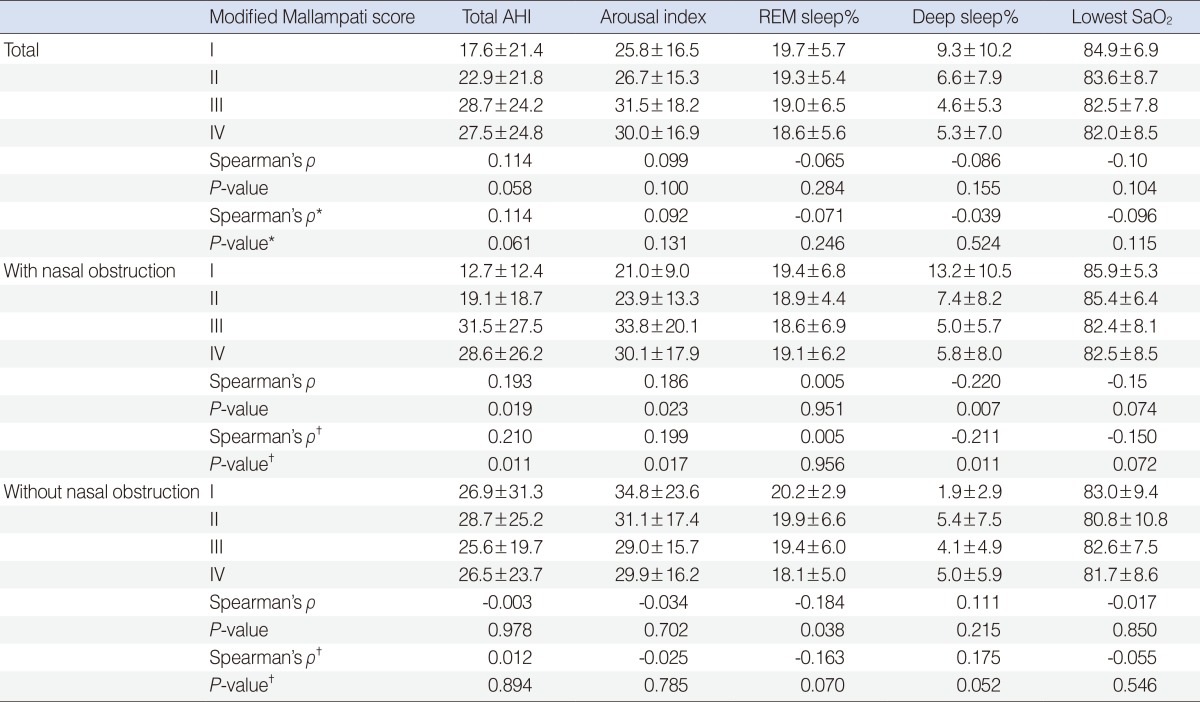 Variable are presented as mean┬▒SD. AHI, apnea-hypopnea index; REM, rapid eye movement. *Analysis using partial sprearman correlation with adjustment for age, sex, tonsillar grade, and presence of nasal obstruction. ŌĆĀAnalysis using partial sprearman correlation with adjustment for age, sex, and tonsillar grade. |
|
||||||||||||||||||||||||||||||||||||||||