INTRODUCTION
Nasal polyps are a prevalent medical condition, affecting 1-4% of the population. Controversies persist regarding the management of nasal polyps because the exact etiology remains unclear. Corticosteroids are beneficial in reducing the size of polyps and preventing recurrences, but proper control of nasal polyposis is a challenging task in clinical situations. It has been reported that the recurrence rate is as high as 60% in patients with severe nasal polyposis (1). Nasal polyps are characterized by stromal edema and increased inflammatory cells. Hyperabsorption of Na+ and increased Cl- permeability may account for stromal edema formation (2).
There are two main families of purinergic receptors (adenosine and P2 receptors). The P2 receptors can in turn be divided into two families (ligand-gated P2X and the G protein-coupled P2Y receptors). To date, seven P2X receptors (P2X1-7) and eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11-14) have been cloned and characterized, and are accepted as valid members in mammals (3). The activation of chloride secretion via purinergic receptors has been reported in the ciliated respiratory epithelial cells cultured from nasal polyps (4). On the other hand, there are reports that the nitric oxide (NO) inhibits Na+ absorption in rat cortical collecting ducts (5) and rat distal lung epithelium (6), while NO activates the cystic fibrosis transmembrane conductance regulator (CFTR) current in human T lymphocytes (7). The total amount of NO is higher in polyps than normal mucosa (8), and inducible nitric oxide synthase (iNOS) is highly expressed in nasal polyps (9). The aim of the present study was to examine the effects of ion transport modulators, including purinergic agonists, NO donors, and the CFTR chloride channel activator on nasal polyps in situ using a voltage-sensitive vibrating probe technique.
MATERIALS AND METHODS
The polyps were freshly obtained from patients with chronic sinusitis during endoscopic sinus surgery and were immediately delivered to the laboratory in cold physiologic saline. No local anesthetic agents were introduced before extirpating the polyps. The submucosa of the polyp was removed under a stereomicroscope and the tissue was divided into several small pieces. The tissue was folded with the apical membrane of the surface ciliated epithelium facing outward and transferred into a recording chamber. Our Institutional Committee approved the use of these tissues with informed consent.
The vibrating probe is a technique for measuring extracellular electrical currents that are steady or slowly changing. The diameter of the vibrating probe tip is about 20 µm and allows the detection of voltages in the low nanovolt range; vibration between two positions within the line of current flow yields voltages that correspond to current flow through resistive physiologic saline. The technique has been described in detail elsewhere (10). Briefly, the short circuit current (Isc) was monitored by vibrating a platinum-iridium wire microelectrode that was insulated with parlene-C (Micro Electrodes, Gaithersburg, MD, USA) and which had been coated with platinum black on the exposed tip. The vibration was about 20 µm along both a horizontal (X) and vertical (Z) axis. The X-axis was perpendicular to the face of the epithelium. The probe was positioned 30-40 µm from the apical surface of the epithelium with computer-controlled, stepper-motor manipulators (Applicable Electronics, Forestdale, MA, USA) and specialized probe software (ASET, version 2.0, Science Wares, East Falmouth, MA, USA). The bath references were 26-gauge platinum-black electrodes. Calibration was performed in physiologic saline (see below) using a glass microelectrode (tip, <1 µm OD) filled with 3 M KCl as a point source of the current. The frequencies of vibration were in the range of 300-400 Hz and were well-separated for the two orthogonal directions. The signals from the oscillators driving the probe were also fed to a dual channel phase-sensitive detector. The signal of the X and Z detectors were connected to a 16 bit analog to digital converter (CIO-DAS1602/16, ComputerBoards, Mansfield, MA, USA) in a Pentium IV computer. The sampling interval was 0.6s, which is the minimum in this software. The electrode was positioned such that Isc showed a maximum X value and a minimum Z value; data are expressed as the vector length of the current density and plotted with Origin software, version 6.1 (OriginLab Software, Northampton, MA, USA). The output from the vibrating probe depends not only on the specific short circuit current of the epithelium, but also on the position of the probe from the surface of the tissue and the exact geometry of each tissue sample. The current density reported here refers to the flux at the position of the probe and represents only a fraction of the current crossing the epithelium. No changes in the relative position of the probe due to swelling or shrinking of the tissue during experimental treatments were observed.
In all experiments, both sides of the polyp were perfused with physiologic saline containing (in mM) 130 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 5 glucose, and 10 HEPES (pH 7.4). Adenosine 5'-triphosphate (ATP), uridine 5'-triphosphate (UTP), 2'- and 3'-O-(4-benzoyl-benzoyl)adenosine 5'-triphosphate (BzATP), α,β-methyleneadenosine 5'-triphosphate αβmeATP), 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid disodium salt hydrate (DIDS), L-arginine, sodium nitroprusside (SNP), and 8-(p-sulfophenyl) theophylline (8-SPT) were directly dissolved in physiologic saline just before use. Uridine 5'-diphosphate (UDP) was pre-incubated for 1.5 h with hexokinase (1 unit/mL) and glucose (5 mM) because the commercial preparations of UDP may be supplied with a minor component of UTP (11). Amiloride, N-phenylanthranilic acid (DPC), genistein, and ouabain were dissolved in DMSO and then diluted to 0.1% DMSO in the control solution prior to application. DMSO at this concentration had no effect on the Isc.
Each tissue was mounted in a perfusion chamber on the stage of an inverted microscope and continuously perfused at 37℃ at an exchange rate of 0.7 times/sec. Ouabain (10 µM), an inhibitor of Na+, K+-ATPase, was tested to estimate the time for the perfusate to migrate to the basolateral membrane of the surface epithelium. Application of ouabain decreased the Isc from 8.6±2.5 min (n=15), which indicates that the ion channel modulators used here acted in a short time on the apical membrane of surface epithelium.
For analysis, the peak Isc was chosen. Data are expressed as the mean±S.E.M. (n=number of tissues) of the Isc. An increase or decrease in Isc were considered significant at a level of P<0.05. A paired t-test was used.
RESULTS
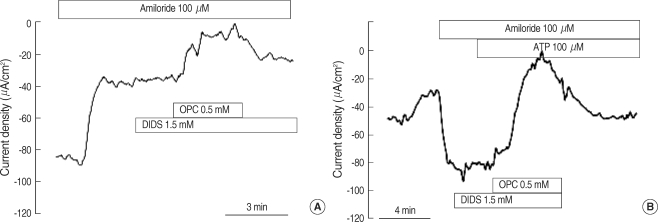
The Isc measured in physiologic saline was -124.0±17.0 µA/cm2 (n=33). Perfusion of amiloride (100 µM), an epithelial sodium channel (ENaC) blocker, significantly decreased the Isc by 78.7±3.1% (to -18.8±2.6 µA/cm2, n=33). In the presence of amiloride, DIDS (1.5 mM) slightly decreased the Isc by 11.1±2.5% (n=17) and subsequent application of DPC (0.5 mM) significantly decreased the Isc by 88.0±2.6% (n=17, Fig. 1A). In the presence of amiloride, 100 µM ATP significantly increased the Isc by 460.1±171.8% (n=7), which was inhibited by DPC (92.1±1.8%), but not by DIDS (17.9±6.7%, Fig. 1B).
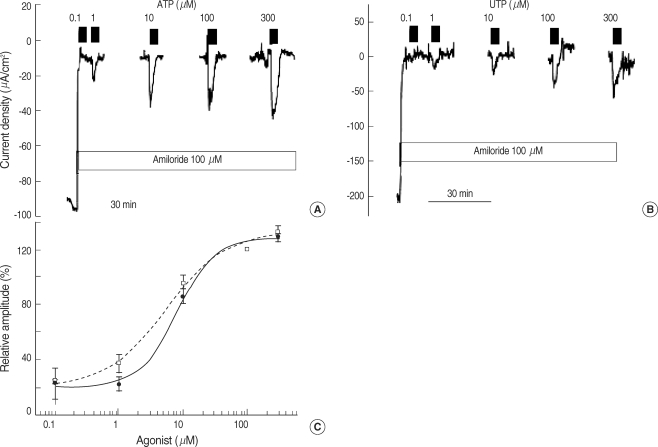
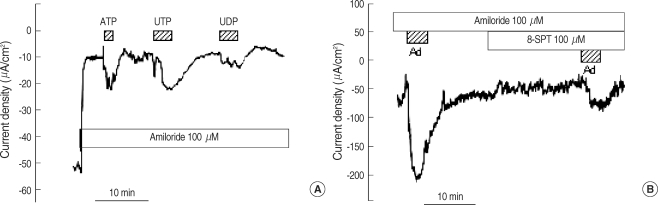
ATP and UTP increased the Isc in a dose-dependent manner (Fig. 2). The EC50 of ATP and UTP was 5.3±0.3 (n=5) and 7.7±0.8 µM (n=5), respectively. UDP, a P2Y6 receptor agonist, also increased the Isc to 44.5±8.9% of the UTP response (n=5, Fig. 3A). Adenosine, a purinergic receptor type 1 (P1) receptor agonist, increased the Isc by 340.1±167.9% (from -21.4±6.6 to =50.0±5.5 µA/cm2, n=7, Fig. 3B). In the presence of 100 µM 8-SPT, a P1 receptor blocker, the response of adenosine was decreased by 90.0±3.7% (from -50.0±5.5 to -23.4±6.5 µA/cm2, n=7, Fig. 3B).
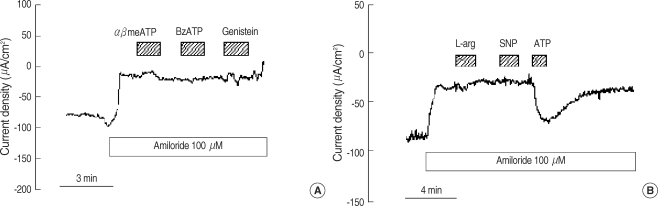
P2X receptor agonists and other modulators for ion transport were introduced to test the effects on polyps (Fig. 4). The addition of αβmeATP and BzATP (100 µM) had no significant response in the presence of amiloride (from -13.2±4.5 to -13.9±3.9 µA/cm2 and -12.7±2.5 to -12.4±2.5 µA/cm2, respectively, n=5 each, Fig. 4A). The addition of genistein (20 µM), the CFTR activator (12), had no effect on the Isc (from -14.1±2.5 to -14.4±2.9 µA/cm2, n=6, Fig. 4A). Perfusion of L-arginine (1 mM, substrate for nitric oxide synthase, n=5) and sodium nitroprusside (1 mM, nitric oxide donor, n=7) showed no response in the absence (-101.7±20.7 µA/cm2 to -95.8±22.3 µA/cm2, -122.1±19.6 µA/cm2 to -118.8±20.5 µA/cm2, respectively) and presence (from -19.8±4.6 to -18.9±4.3 µA/cm2, -18.8±4.6 to -18.9±4.3 µA/cm2, respectively, Fig. 4B) of amiloride.
DISCUSSION
The Isc measured in polyps largely represents the current from the ciliated epithelial cells because the superficial epithelium of the polyp is characterized by ~80% ciliated cells (13). The blockade of Na+ absorption by amiloride is consistent with a previous report involving nasal polyps in situ (14). In the presence of amiloride, DIDS had little effect on the Isc, but DPC abolished the Isc. Although DIDS and DPC are non-specific anion channel blockers, current pharmacology indicates that DIDS does not act on the CFTR chloride channel, even at high concentrations (15), and DPC induces a voltage-dependent blockade of CFTR when used at micromolar concentrations (16). Therefore, after the addition of amiloride the Isc is likely to be dominated by CFTR-mediated chloride conductance. ATP strongly stimulated DPC-sensitive chloride secretion and its potency was similar to UTP. This is consistent with the activation of the human P2Y2 receptor (17) because ATP acts as a competitive antagonist on the human P2Y4 receptor (18). The EC50 of ATP and UTP measured in this study was 5.3 and 7.3 µM, respectively, which was similar to the EC50 reported in cultured cells in terms of anion transport (ATP, 1.0 µM) (19) and ciliary beat frequency (UTP, 4.7 µM) (20). UDP, the principal agonist for P2Y6, increased the chloride secretion to 44% of the UTP response, which is similar magnitude in magnitude in terms of ciliary beat frequency (20). This finding indicates that the P2Y6 receptor is likely to be active. Besides, we have eliminated the possibility of UTP contamination from UDP preparation by addition of hexokinase and glucose. This point was not clearly noted in the previous reports concerning P2Y6 characterization in the airway epithelium (20). Another P1 purinergic agonist, adenosine, also activated chloride secretion, which was inhibited by a non-specific adenosine receptor blocker, 8-SPT (21).
We examined further how much P2X receptors are functionally implicated in nasal polyp using αβmeATP and BzATP. The αβATP is an agonist for P2X1 and P2X3, and BzATP is for P2X1, P2X2, and P2X7 (22). P2X receptors could not be detected pharmacologically, which indicates that P2X receptors do not have a significant role compared to P2Y receptors. This is contrary to the report which described these agonists having large responses in lung-derived cultured cells (23). The CFTR activator, genistein (24), did not induce a CFTR current, which may be attributed to an altered localization of CFTR in the nasal polyp (25). If NO, generated by the application of NO substrate (L-arginine) or NO donor (SNP), has a regulatory effect on the CFTR or ENaC, it can serve as a medical, therapeutic strategy for nasal polyps. However, there was no beneficial effect at high concentrations of L-arginine and SNP, both in the absence and presence of amiloride in nasal polyps, which is similar to the observation in cultured human nasal epithelium (26).
In conclusion, the multiple P2Y receptors were functioning at the apical membrane of the nasal polyp in situ. However, P2X agonists, the CFTR activator, and NO-producing drugs did not have a beneficial effect on nasal polyps in situ.