|
 |
- Search
AbstractObjectivesBone-anchored hearing device (BAHD) is contraindicated in patients younger than 5 years because their calvarial bones are not thick enough to be implanted site. However, it has not been studied in the Korean population. This study was not only to establish a safe guideline for depth of implant device in all age groups who undergo BAHD implant surgery, but also to investigate whether implantation of currently used BAHDs could be done safely in Korean children, especially those younger than 5.
MethodsTwo hundred eighty patients, who underwent high-resolution temporal bone computed tomography (TBCT) images between August 2010 and October 2018 were randomly enrolled in all ages. We retrospectively reviewed TBCT imaging to measure skull bone thickness at the recommended BAHD implant site.
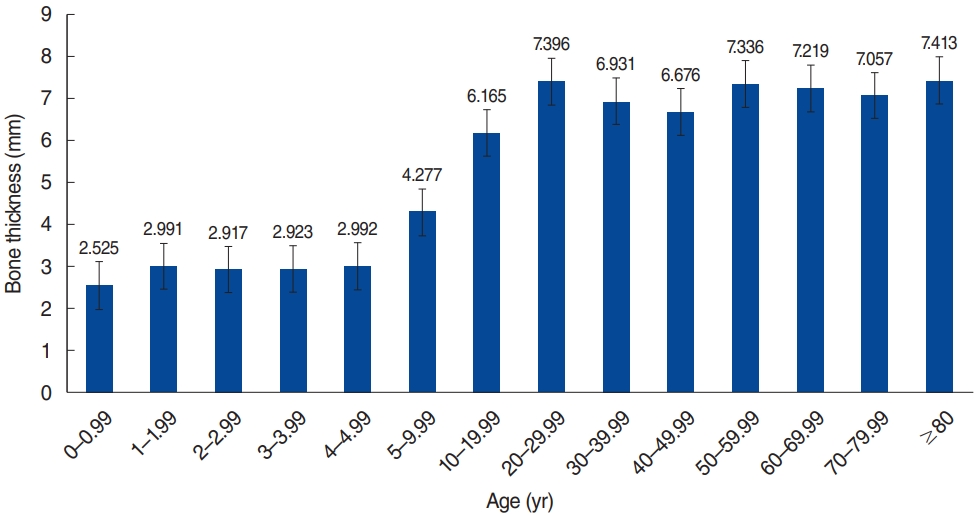
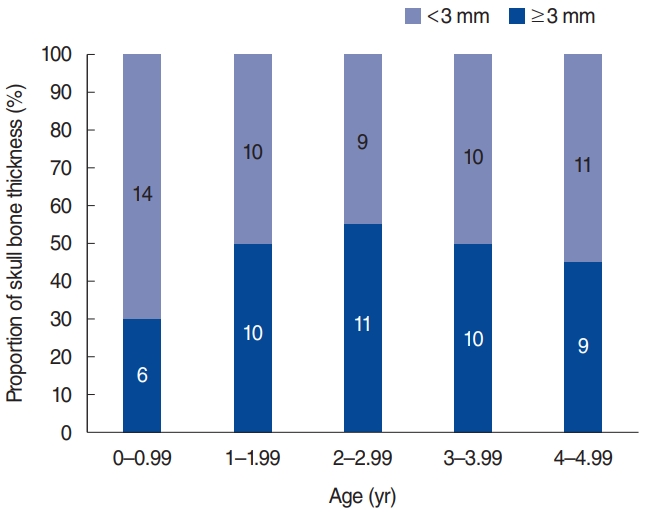
ResultsThe average skull bone thickness was 2.87 mm in patients younger than 5 years and 6.72 mm in patients older than 5 years, respectively, which conforms to the current guideline. The results indicate nearly 50% of calvarial bone thicknesses were less than 3 mm in patients under 5 years old, while 92.78% of the patients older than 5 years of age showed bone thickness greater than 4 mm. Of note, calvarial bone thickness was thicker than 3 mm in all patients who are older than 6 years.
Bone conduction devices (BCDs) have improved hearing for patients who have hearing loss when conventional air conduction hearing aids are inappropriate [1]. Conventional BCDs were developed in the beginning of the 20th century with a sound processor attached with spectacles, steel spring headbands, or soft headbands [2]. Osseo-integrated titanium implants were successfully introduced into clinical field of otology in the late 1970s by Tjellstrom et al. [3]. This new percutaneous BCDs transmits vibrations through screw directly to the bone instead of through the skin, and the high frequencies can be preserved [4]. But it still has drawbacks such as loss of osseointegration or skin complications. Since then, implantable BCDs have newly developed over recent years [5]. To conduct sound directly to the cochlea, a titanium prosthesis is implanted into the calvarium, and there are two ways to attach an external processor (receiver) to the implant, via a percutaneous abutment or via transcutaneous magnetic attraction [6]. Although percutaneous types, such as the Baha Connect (Cochlear Corp., Sydney, Australia), Bone Bridge (Med-El, Innsbruck, Austria), and Ponto (Oticon Medical, Askim, Sweden), have 5 to 7 dB more auditory gain due to direct coupling of the processor (receiver) to the implant, transcutaneous types, like the Sophono (Medtronic, Dublin, Ireland) and Baha Attract (Cochlear Corp.), are cosmetically better and safer with fewer soft tissue complications [6].
The audiological indications for osseointegrated auditory devices include unilateral or bilateral conductive or mixed hearing loss [6]. Single-sided sensorineural deafness can be also a good candidate for this type of device that stimulates contralateral cochlea [7]. In addition, hearing loss patients with different kinds of etiologies including congenital, acquired, infectious, neoplastic, traumatic, and iatrogenic can be included as medical indications [8], especially in pediatric patients, although early hearing rehabilitation for pediatric hearing loss is very important and contributes to better social skills as well as better linguistic and academic outcomes [9]. Currently established bone conduction implant (BCI) guidelines, as approved by the U.S. Food and Drug Administration, suggest patients should be older than 5 years [10].
When surgically implanting BCDs for indicated patients, it is important to safely drill a deep hole without mechanically damaging the dura mater, as a hole with proper depth may improve osseointegration. The most common devices such as Bonebridge (Med-El), Ponto (Oticon Medical), and Baha Connect (Cochlear Corp.) need a 3 or 4 mm depth hole for implantation. Especially in children, bone thickness is an essential factor to consider and this is one of the reasons why there is no current approval for bone-anchored hearing device (BAHD) use in patients younger than 5 years of age. To investigate whether the age guideline is reasonable, one U.S. group measured temporal bone thickness in pediatric patients and showed that pediatric temporal bone thickness in patients under 5 years old is generally thicker than 3 mm in Caucasians [11]. However, there have been no specific studies investigating skull bone thickness for BAHDs in Korean populations.
This study aimed to investigate the guideline regarding safe implant device depth in all age groups who undergo bone conductive implant surgery by reviewing bone thickness at implant site with computed tomography (CT) imaging. We also aimed to investigate whether implantation of currently used BCI devices is safe in pediatric Korean group, especially patients younger than 5 years. Thus, this is the first Korean study investigating skull bone thickness at the BCI implant site and we validated the current guideline in a Korean sample.
Two hundred eighty patients, who underwent temporal bone CT images between August 2010 and October 2018 were randomly collected across all ages. We retrospectively reviewed high-resolution temporal bone computed tomography (TBCT) imaging. The study was in accordance with common ethical rules and was approved by Samsung Medical CenterŌĆÖs Institutional Review Board (IRB No. 2019-05-034). Patients groups were stratified by age and each group consisted of 20 subjects. For ages from 0 to 4.99 years, groups were divided by 1 year, and ages from 5 to 9.99 years were categorized as the next group. For ages of 10 years or more, seven groups were divided by decade, and the last group comprised patients older than 80. The 280 patients consisted of 132 females and 148 males. In this study, patients who had congenital bone anomaly or history of skull bone fracture were excluded. We also excluded all patients who had a specific disease in the ear or had previous otology surgery. One neuro-otologist measured the skull bone thickness on the axial image at a point 1 cm posterior to the right sigmoid sinus and 1 cm superior to the superior margin of the right external auditory canal, where BAHA implantation is recommended (Fig. 1) [11,12]. Results were analyzed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA). The chi-square analysis was performed to determine how the skull thickness (<3 mm or Ōēź3 mm) differs according to age. Changes in skull thickness with age were analyzed using simple linear regression model.
In this study, a total of right side 280 ears were reviewed. One hundred thirty-two patients were female and 148 patients were male. Measured skull bone thickness average by age group is presented in Fig. 2. In this Korean sample, we found that the average skull bone thickness was 2.87 mm for patients under 5 years old and 6.72 mm for patients over 5 years old. In groups younger than 5 years of age, we also compared the number of patients whose bone thickness was less than 3 mm to those whose bone thickness was more than 3 mm in Fig. 3. We found that averagely 54% of patients had a skull bone thickness that was thinner than 3 mm among the groups younger than 5 years of age. However, the chi-square analysis of each age did not show statistical differences at all ages.
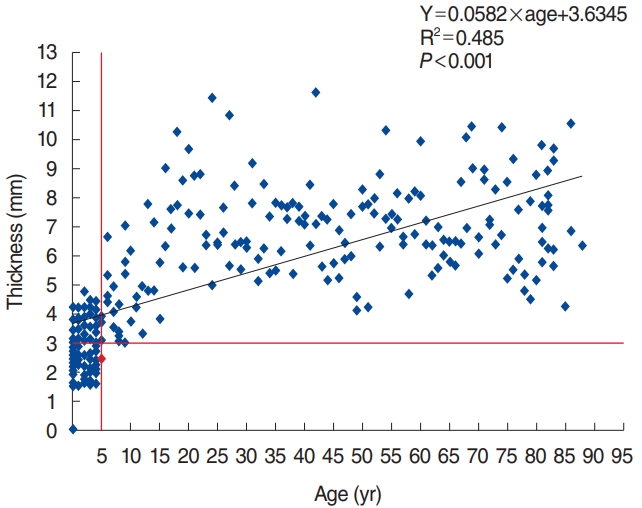
The measured thickness is plotted by age in Fig. 4 and the trend line shows a linear correlation between thickness and age (thickness=0.0582├Śage+3.6345, R┬▓=0.485, P<0.001). All measured thicknesses in patients who were older than 6 years were larger than 3 mm and only one 5-year-old patientŌĆÖs bone thickness was less than 3 mm. This showed that 92.78% (167 patients) of patients had a skull thickness that was greater than 4 mm.
Most BAHDŌĆÖs surgical guidelines indicate that calvarial bone thickness should be greater than 3 mm, as the shortest fixtureŌĆÖs length is 3 mm. Furthermore, as the standard fixtureŌĆÖs length is 4 mm, calvarial bone thickness of more than 4 mm is important in planning BAHD implantation. One previous study investigated temporal bone thickness in Caucasians to assess the benefit of BAHD implantation in children younger than 5 years of age. The result of that study showed the average squamous temporal bone thickness was thicker than 3 mm in all age groups, which means that BAHD implantation could be considered for patients younger than 5 years of age [11]. However, no previous studies have investigated BAHD indications with respect to calvarial bone thickness in Korean groups and the present study is the first to establish a safe guideline for Korean groups.
In this study, the average skull bone thickness was thinner than 3 mm in age groups under 5 years and greater than 4 mm in age groups older than 5 years in a Korean sample (Fig. 2). This result is different from the previous study in Caucasians, suggesting that BAHDs should not be routinely applied to patients younger than 5 years of age [11]. Our results are in line with many previous studies showing that skull bone thickness is less than 3 mm in patients younger than 5. A retrospectively reviewed study of pediatric patients with unilateral BAHD measured 29 patientsŌĆÖ bone thickness, and the measured thickness varied from 1 to 4 mm (mean┬▒standard deviation [SD], 2.5┬▒ 0.8 mm) when the average age was 8.4┬▒4.6 years, which is even older than 5 years [13]. Another study measured bone thickness in 68 children during BAHD implantation surgery and it was found to vary from 0.5 to 5 mm (mean┬▒SD, 2.5┬▒0.8 mm). They also proposed that bone thickness rapidly increases during the first 5 years, and appropriate bone thickness for implants would be reached between 5 to 7 years [14].
In addition, Fig. 3 shows that nearly 50% of patientsŌĆÖ bone thickness was less than 3 mm in patients younger than 5 years of age. This implies that even if the average of skull bone thickness is greater than 3 mm, there may be many patients whose bone thickness is less than 3 mm. Therefore, even if the average temporal bone is greater than 3 mm in a Caucasian population, this high average never guarantees the safety of BAHD implantation in all patients younger than 5 years of age.
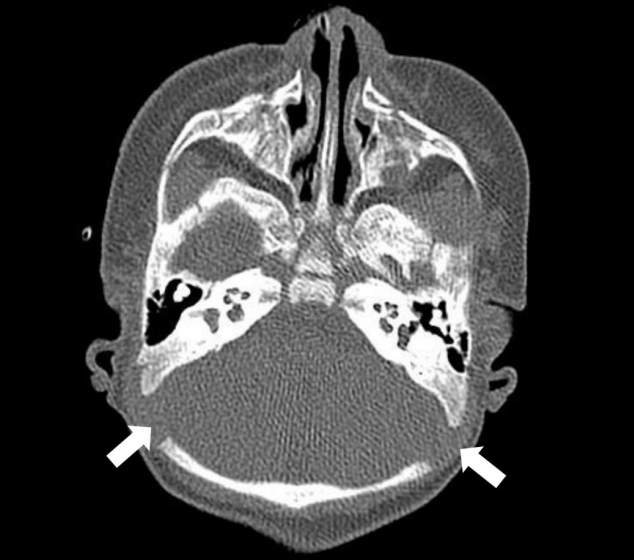
There are many reasons why we discuss considering age and bone thickness at the implant site when applying BAHD in this study. Although it has been shown that BAHD is highly advantageous, there can be problems when applying it to thin skull bone. Therefore, we need to evaluate the potential risk of thin skull bone prior to implantation in patients whose bone thickness is less than 3 mm before surgery. At first, we can intuitively think that trauma can easily occur with thinner bone during surgery and thinner bone is more vulnerable compared to thicker bone. In this study, there was one patient whose fontanel had not closed which was a critical condition (Fig. 5). In addition, the dura or sigmoid sinus can be easily exposed during surgery. According to previous studies targeting pediatric patients, 39% of implants were installed in contact with the dura, sigmoid sinus, or an air cell [14]. Another study reported that the dura and sigmoid sinus were exposed during BAHD implant surgery in 70.5% of pediatric cases [13]. These exposures increase the risk of cerebrospinal fluid leak and extradural hematoma. Furthermore, the degree of osseointegration and stability are lower in thinner bone compared to thicker bone, which can cause implantation failure. A recent study evaluated BCI stability in children by age and bone thickness during subsequent visits after surgery. The results showed that the thicker the bone, the higher the obtained implant stability quotient value [15]. In addition, in the Birmingham BAHD program, 39 children were implanted before their fifth birthday, and the fixture failure rate was high in this group (40%) [16]. Furthermore, previous investigators reported that younger age was independently associated with a higher rate of osseointegration failure after assessing 68 pediatric patients in the age range of 5 to 19 years (average, 10.98 years) [17]. Although many other reasons may contribute to higher risk of osseointegration failure in a younger population, thin bone thickness plays a major role.
As we discussed above, implantation of BAHDs in patients younger than 5 years of age is not recommended mainly due to thin skull bone, we must not dismiss them due to the need for early hearing rehabilitation. In these very young patients, noninvasive options can be tried, including Baha Softband (Cochlear Corp.), Ponto Softband (Oticon Medical), Baha SoundArc (Cochlear Corp.), or ADHEAR (Med-El) as a first step until bone has grown sufficiently. Those nonimplanted BCDs are commonly designed for all ages including infants and toddlers who are not yet ready for implantation. By wearing this application, young patients can have better hearing performance and enough amplification to facilitate language development. Recent studies have shown the Softband option is beneficial for aural rehabilitation in these very young patients [16]. One study retrospectively reported that the hearing ability of children with bilateral congenital aural atresia improved significantly after using the Baha Softband. This study also showed that wearing the Softband at a young age could guarantee relatively normal hearing development and attenuated oral communication impairment [18].
In addition, a few patients might not show sufficient bone thickness even if they are older than 5 years of age. We found that one 5-year-old patient had a calvarial bone thickness less than 3 mm. Therefore, for safety, we need to check with TBCT to evaluate bone thickness before surgery in pediatric populations. If calvarial bone at the implant site is thinner than 3 mm, delaying surgery and applying noninvasive BCDs are needed until the skull bone grows enough. Moreover, bone growth stimulation with a barrier membrane might be another solution that can be tried. Bone augmentation was first described by Granstrom and Tjellstrom [19]. To stimulate bone growth, expanded polytetrafluoroethylene is positioned under the flange of the fixture in this process and this barrier membrane supports bone generation to produce sufficient bone for implantation [19].
On the other hand, some very young patients already have sufficient bone thickness before 5 years of age as shown in Fig. 3. However, we must not implant BAHD only depending on bone thickness. Although we have proposed that there is a correlation between skull bone thickness and age, and bone thickness is major factor to consider when we implant BAHDs, skull bone thickness is not the sole reason why we cannot implant BAHD in patients younger than 5 years of age. We need to consider other age-limiting factors as well. The infant skull is lower in minerals and higher in water content compared to the adult skull and this fact supports that osseointegration runs a higher risk of failure in younger populations [10]. Therefore, the benefit and the loss for implantation in very young patients should be carefully assessed. Similarly, we also need to compare the pros and cons between early implantation of BAHD and application of noninvasive bone-conduction options until the age of 5 in very young patients who have sufficiently thick calvarial bones.
This study also showed noticeable results regarding calvarial bone thickness in adult groups. According to the result presented in Fig. 4, it is remarkable to see that 92.78% of their skull bone thickness was greater than 4 mm among patients who were older than 5 years of age (Fig. 4), and all measured thicknesses in patients who were older than 6 years old were thicker than 3 mm. This result implies that we can safely apply BAHD with a 3 mm fixture in Korean adult groups. It also indicates that a 4 mm-fixture can be implanted in most adult patients safely. Furthermore, the averages bone thickness in groups older than 10 years old was thicker than 6 mm, which is significantly larger than the current fixture length of 4 mm. This suggests the need for longer fixtures than 4 mm to obtain better stability and osseointegration for patients who have sufficient skull bone thickness. But, in case of Bonebridge, floating mass transducer position and screw fixation could be performed inside sinodural angle. Though the cortical bone of this area is usually thinner than the measured point in this study, so pay attention to the position and fixation, also consider other location.
We conducted a retrospective review of a large set of TBCT data to assess calvarial bone thickness at the BAHD implantation site in all age groups in a Korean sample. The results indicate guidelines for BAHD implantation are appropriate in a Korean population when we only consider bone thickness. Likewise, current age indication for BAHD, the average skull bone thickness was less than 3 mm in patients younger than 5 years of age and greater than 3 mm over 5 years of age. For children younger than 5 years of age, applying noninvasive devices to promote their early hearing rehabilitation is necessary until they get sufficient bone thickness around the age of 5 years. Furthermore, we recommend performing TBCT prior to surgery, especially in pediatric patients, to ascertain bone thickness. In addition, in adult groups, since they have enough skull bone thickness to apply longer fixtures, thicker fixture options by manufacturers should be considered.
Ō¢¬ The currently approved bone-anchored hearing device implantation guideline is suitable in the Korean population.
Ō¢¬ We suggest taking temporal bone computed tomography before surgery for safety, especially in pediatric patients.
Ō¢¬ Noninvasive applications are recommended for patients younger than 5 years of age.
NotesAUTHOR CONTRIBUTIONS Conceptualization: YSC, IJM. Data curation: SK, YSC. Formal analysis: SK. Methodology: SK, YSC. Project administration: IJM. Visualization: SK. WritingŌĆōoriginal draft: SK. WritingŌĆōreview & editing: YSC, YSC, IJM. Fig.┬Ā1.Axial slice of temporal bone by computed tomography and example of skull bone thickness is marked at the point of measurement. Measurement point is 1 cm posterior to the right sigmoid sinus posterior margin (black arrow), and 1 cm superior to the superior margin of the right external auditory canal. 
REFERENCES1. Powell R, Wearden A, Pardesi SM, Green K. Understanding the low uptake of bone-anchored hearing aids: a review. J Laryngol Otol. 2017 Mar;131(3):190-201.
2. Mudry A, Tjellstrom A. Historical background of bone conduction hearing devices and bone conduction hearing aids. In: Kompis M, Caversaccio MD, editors. Implantable bone conduction hearing aids. Basel: Karger Publishers; 2011. p. 1-9.
3. Tjellstrom A, Lindstrom J, Hallen O, Albrektsson T, Branemark PI. Osseointegrated titanium implants in the temporal bone: a clinical study on bone-anchored hearing aids. Am J Otol. 1981 Apr;2(4):304-10.
4. Reinfeldt S, Hakansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 2015 Jan;8:79-93.
5. Doshi J, McDermott AL. Bone anchored hearing aids in children. Expert Rev Med Devices. 2015 Jan;12(1):73-82.
6. Kohan D, Ghossaini SN. Osseointegrated auditory devices-transcutaneous: Sophono and Baha attract. Otolaryngol Clin North Am. 2019 Apr;52(2):253-63.
7. Kim G, Ju HM, Lee SH, Kim HS, Kwon JA, Seo YJ. Efficacy of boneanchored hearing aids in single-sided deafness: a systematic review. Otol Neurotol. 2017 Apr;38(4):473-83.
8. Svrakic M, Vambutas A. Medical and audiological indications for implantable auditory devices. Otolaryngol Clin North Am. 2019 Apr;52(2):195-210.
9. Alzahrani M, Tabet P, Saliba I. Pediatric hearing loss: common causes, diagnosis and therapeutic approach. Minerva Pediatr. 2015 Feb;67(1):75-90.
10. Davids T, Gordon KA, Clutton D, Papsin BC. Bone-anchored hearing aids in infants and children younger than 5 years. Arch Otolaryngol Head Neck Surg. 2007 Jan;133(1):51-5.
11. Baker A, Fanelli D, Kanekar S, Isildak H. A review of temporal bone CT imaging with respect to pediatric bone-anchored hearing aid placement. Otol Neurotol. 2016 Oct;37(9):1366-9.
12. Baker AR, Fanelli DG, Kanekar S, Isildak H. A retrospective review of temporal bone imaging with respect to bone-anchored hearing aid placement. Otol Neurotol. 2017 Jan;38(1):86-8.
13. Priwin C, Granstrom G. The bone-anchored hearing aid in children: a surgical and questionnaire follow-up study. Otolaryngol Head Neck Surg. 2005 Apr;132(4):559-65.
14. Granstrom G, Bergstrom K, Odersjo M, Tjellstrom A. Osseointegrated implants in children: experience from our first 100 patients. Otolaryngol Head Neck Surg. 2001 Jul;125(1):85-92.
15. Mierzwinski J, Konopka W, Drela M, Laz P, Smiechura M, Struzycka M, et al. Evaluation of bone conduction implant stability and soft tissue status in children in relation to age, bone thickness, and sound processor loading time. Otol Neurotol. 2015 Aug;36(7):1209-15.
16. McDermott AL, Williams J, Kuo M, Reid A, Proops D. The birmingham pediatric bone-anchored hearing aid program: a 15-year experience. Otol Neurotol. 2009 Feb;30(2):178-83.
17. Nelson KL, Cox MD, Richter GT, Dornhoffer JL. A comparative review of osseointegration failure between osseointegrated bone conduction device models in pediatric patients. Otol Neurotol. 2016 Mar;37(3):276-80.
|
|
|||||||||||||||||||||||||||||||||||||||||||