 |
 |
- Search
AbstractObjectivesPost-radiation nasopharyngeal necrosis (PRNN) is a serious complication that severely impacts the quality of life and survival of nasopharyngeal carcinoma patients. Endoscopic debridement is considered the first-line treatment for PRNN. This study aimed to analyze clinical outcomes, focusing on the mucosal resurfacing status and the effectiveness of salvage operations.
MethodsTwenty-seven patients who underwent endoscopic debridement were retrospectively analyzed. The patients were divided into two groups according to the initial surgical modality: debridement with a nasoseptal flap (NSF; n=21) and debridement only (no NSF; n=6). Clinical features, postoperative mucosal status, internal carotid artery (ICA) rupture, survival, and final mucosal status were evaluated. The NSF group was categorized according to flap viability to analyze risk factors for flap failure.
ResultsRegardless of the initial modality, most patients experienced symptom improvement (96.0% for headache and 100% for foul odor); however, complete cranial nerve palsy did not improve in any patients. In the NSF group, complete healing was observed in 66.7%, while all patients in the no-NSF group underwent salvage surgery because none maintained complete healing. In the NSF group, 19.0% of patients required salvage surgery. After the last operation, favorable symptom improvement was noted (100% for headache and 90.0% for foul odor), and 77.8% had completely healed mucosa, whereas only 14.8% and 7.4% had partial healing and persistent necrotic mucosal status. The necrotic or uncovered NSF subgroup showed statistically non-significant tendencies for old age, advanced necrosis stage, advanced T stage, ICA involvement, high frequency and dose of radiation therapy, diabetes mellitus, and underlying comorbidities. Two ICA ruptures and three deaths occurred.
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in the head and neck area, especially in East and Southeast Asia [1,2]. Radiation therapy (RT) either with or without chemotherapy is the mainstay treatment for NPC, and the 5-year overall survival (OS) rates vary from 63.0% to 87.4% due to advancements in RT technique and the application of adjuvant chemotherapy in locoregionally advanced NPC patients [3-6]. Although RT has a high response rate and shows favorable survival, it can induce several severe complications, such as postradiation nasopharyngeal necrosis (PRNN) [7-9]. Although it is not a common complication, with an incidence rate of 0.8%–1.1% [8,10], PRNN can affect the nasopharyngeal mucosa, parapharyngeal tissues, or skull base, thereby inducing severe headache, foul odor, cranial nerve palsy (CNP), and even massive bleeding due to internal carotid artery (ICA) rupture, which can seriously impact patients’ quality of life and survival [8,9,11-13].
Therefore, timely diagnosis and proper management of PRNN are essential, and endoscopic debridement has shown favorable outcomes with low morbidity [7,8]. A vascularized flap is needed to cover the exposed ICA or dura to reduce the risk of catastrophic events such as carotid blow-out or meningitis. Alongside our previous study [12], several recent studies have reported promising outcomes regarding the use of a nasoseptal flap (NSF) to resurface the defect site, showing improved functional outcomes and better OS [10,14-16]. Although promising results have been obtained using NSFs, there are still cases of failure and incomplete resolution of PRNN. Nonetheless, little has been explained regarding the mechanism of this complication and there is a lack of research on how to treat such intractable cases after failed NSF reconstruction. Thus, focusing on the mucosal resurfacing status of nasopharyngeal wounds, our study aimed to analyze the clinical outcomes of flap reconstruction after endoscopic debridement based on the mucosal resurfacing status and to investigate the effectiveness of salvage operations.
We retrospectively reviewed the medical records of NPC patients who were surgically treated for PRNN at our institution between April 2013 and March 2021. We selected patients for surgical treatment based on radiation history for NPC, clinical features (including headache, foul odor, and CNP), endoscopic findings, and radiologic studies (such as computed tomography [CT] and magnetic resonance imaging [MRI]). A total of 31 patients underwent endoscopic debridement for PRNN. Among them, four patients with pathologically confirmed recurrent tumor were excluded. Finally, 27 patients were analyzed in the present study. This study was approved by the Institutional Review Board of Samsung Medical Center (No. 2022-04-039), and the need for informed consent was waived.
According to the method of initial surgery, patients were divided into two groups, debridement with NSF (NSF group, n=21) and debridement only (no NSF group, n=6). The patients with continuous or relapsed symptoms along with persistently necrotic surgical bed were considered candidates for salvage surgery. ICA involvement, defined as necrotic tissue surrounding the ICA, no healthy tissues above the necrotic tissue over the ICA, and evidence of a narrowing of the ICA lumen in comparison to the contralateral ICA, even after surgery for radionecrosis was also considered for salvage surgery.
Ultimately, salvage surgery was performed in 10 patients with reconstruction using an NSF (n=5), anterolateral thigh free flap (ALTFF, n=4), and inferior turbinate (IT) mucosal free graft followed by middle turbinate rotation flap (MTF, n=1). All patients were regularly followed up at 3- to 6-month intervals until at least 5 years after the initial surgery. Nasopharyngeal endoscopic evaluation, contrast-enhanced CT scans, and MRI were performed as follow-up evaluations.
If the ICA was surrounded with necrotic tissue, we performed the balloon occlusion test (BOT) for potential risk of ICA injury and sacrifice. All patients underwent endoscopic debridement with image-guided surgery. After performing frozen biopsy to exclude recurrent tumor, wide debridement was performed using curettes, a microdebrider or Coblator (Coblator II; Smith & Nephew, Memphis, TN, USA). After debriding the devitalized soft tissue, an endoscopic drill was used to remove the necrotic portion of the skull base bone. Careful attention was paid when debriding on the posterolateral side to avoid carotid artery rupture using Doppler ultrasound. If the carotid artery was surrounded by necrotic tissue, the necrotic tissue was intentionally left in place. After debridement, we performed massive povidone-iodine irrigation.
In the NSF group, we covered the defect with NSF based on the septal branch of sphenopalatine artery. NSF was usually harvested from the contralateral side of the main lesion to avoid potential pedicle injury during debridement around the eustachian tube. The NSF was elevated from the mucocutaneous junction (caudal septal margin), including the nasal floor for a wider width. Gelfoam (Pfizer Inc., New York, NY, USA) pledgets and Merocel (Medtronic Xomed, Jacksonville, FL, USA) nasal packing were used to bolster the area and deter flap detachment. The nasopharynx was packed for 7 to 10 days. Following the removal of the packing, the patients started irrigating the lesion with normal saline solution. Supplementary Video 1 is an example of intraoperative video clip of above endoscopic debridement with NSF reconstruction procedure.
When performing salvage surgery, we collected necrotic tissue and remaining deep tissue for frozen biopsy to exclude tumor recurrence. The following debridement procedure was similar to that of the initial surgery. For reconstruction using ALTFF, we used two approaches, the maxillary swing approach for three patients and the transcervical approach for one patient. Regarding the maxillary swing approach, a Weber–Ferguson–Longmire facial incision was performed to allow a view for further wide debridement and adequate space for transferring the ALTFF. Proper ALTFF donor size and shape were based on defect size and the need for adequate coverage of the ICA. Then, a linear skin incision was created from the anterior superior iliac spine to the lateral border of the patella in the non-dominant leg of the patient. To identify the perforator of the descending branch of the lateral circumflex femoral artery, which is a feeding artery to the ALTFF, we performed dissection through the level of the rectus femoris and vastus lateralis. After identifying the muscular perforators entering the vastus lateralis, the designed fasciomuscular flap was harvested with the perforators. The flap was then transferred to the nasopharyngeal area and sutured to the nasopharyngeal wall with Vicryl suture. This procedure was conducted using the transcervical approach for one patient, where a transverse skin incision was performed on the neck level II area along the skin crease. Arterial anastomoses were performed to the facial artery for three and transverse cervical artery for one, and venous anastomoses to the facial vein for two and external jugular vein and transverse cervical vein each in one patient. Two patients required tracheostomy, while nasopharyngeal airway was inserted in addition to nasal packing and endotracheal tube was maintained till extubation on one day postoperation each in one patient. All procedures described above were performed by ENT surgeons. For one patient who received MTF for a second salvage operation, a posterior lateral nasal artery pedicled posterior-based MTF was designed. An incision was made anterior to the middle turbinate, afterwards mucoperiosteum was elevated from the front aspect, and the middle turbinate bone was removed. It is critical to avoid damaging the vascular pedicle as it enters at its lateral attachment. After the flap was harvested, it was gently rotated to the nasopharynx and bolstered for 7–10 days using Gelfoam and Merocel.
Pre- and postoperative symptoms of headache and foul odor, CNP, postoperative mucosal status, and salvage operation rate were evaluated to compare outcomes according to initial surgical method. Postoperative mucosal status was classified as complete healing, partial healing, or persistent necrosis (Fig. 1).
Within the NSF group, patients were subcategorized into the NSF viable subgroup (NSF-V) and the NSF necrotic or uncovered subgroup (NSF-U) according to flap viability after initial surgery for risk factor analysis of flap failure. The risk of flap failure was analyzed according to age, diabetes mellitus (DM), underlying comorbidity, necrosis stage, T stage, ICA involvement, and RT frequency and dose. Based on the clinical features of patient’ symptom, endoscopic examination and radiological findings, necrosis stage was classified as early, middle, or late, as described in previous studies (Fig. 2) [8,12]. Symptoms of headache and foul odor, CNP, final mucosal status, and ICA rupture, along with mortality rates were evaluated for final outcome measurement.
Patient characteristics are summarized in Table 1. The mean age was 59.6 years (standard deviation, 10.1 years). The median follow-up period was 32 months (range, 1–85 months). The mean number and cumulative dose of RT were 1.6 (range, 1–4) and 9,914 cGy (range, 6,600–21,600 cGy), respectively. The median T stage was 3 (range, 1–4), and all patients underwent concurrent or adjuvant chemotherapy. Regarding symptoms, 25 patients had headaches, 19 had a foul odor, and four had CNP (cranial nerve [CN] 10 for 1, CN 12 for 1, and both CNs 10 and 12 for two patients). On endoscopic examination, 24 patients (88.9%) exhibited late-stage necrosis, and three patients showed middle-stage necrosis. ICA involvement was detected in 13 patients (48.1%). The detailed characteristics of each patient are provided in Supplementary Table 1.
The initial surgery outcomes are summarized in Table 2. Regardless of the initial surgical modality, most patients experienced improvement of symptoms (96.0% for headache and 100% for foul odor); however, those with CNP did not experience any improvement. Furthermore, 14 patients (51.9%) had completely healed mucosa (Fig. 3), whereas five (18.5%) and eight (29.6%) patients had partially healed and necrotic mucosa after initial surgery, respectively. In the NSF group, 66.7% of patients achieved complete healing, while no patients in the no-NSF group maintained complete healing, such that all patients in the no-NSF group needed salvage surgery. Even with the higher rate of ICA involvement in the NSF group (57.1% vs. 16.7%, P=0.086), only four patients required salvage surgery, which was a significantly lower surgical failure rate than in the no-NSF group (19.0% vs. 100%, P<0.001). The mean time until salvage surgery was 9.4 months in both groups, though it had a tendency to be longer in the no-NSF group (13.5 months vs. 3.3 months, P=0.257).
Salvage surgery was performed in 10 patients with recurrent headache and a necrotic surgical bed, with reconstruction using NSFs in five, ALTFFs (Fig. 4) in four, and an IT mucosal free graft in one patient who underwent second salvage surgery with MTF. After salvage surgery, no patients complained of a foul odor, and 90.0% of patients experienced relief from headaches. Moreover, seven (70.0%) had complete healing, whereas two (20.0%) had partial healing, and one patient (10.0%) had persistent necrosis even after salvage surgery. The only patient (case 25) who underwent salvage surgery for a second time had headache relapse due to progression of the partially uncovered mucosal status after the first salvage operation with an IT mucosal free graft; however, the pain improved after the second salvage operation using an MTF with maintenance of fully viable mucosa (the interval between the salvage operations was 50 months). Patient demographics and clinical features in salvage surgery are summarized in Supplementary Table 2.
After their last operation, 21 patients (77.8%) had completely healed mucosa on their last outpatient clinic visit, whereas four (14.8%) and two (7.4%) patients had partial healing and persistent necrotic mucosal status, respectively. No patients reported experiencing a foul odor, and 13 patients (48.1%) still suffered from headaches, but 11 of them noted improvements compared to their initial condition. Ten patients had persistent CNP; two cases were better than the initial status, two cases were worse, and six cases newly developed during follow-up (CN 10 for four; CN 12 for one; both CNs 10 and 12 for four; and CNs 7, 10, and 12 for one patient). The vagus nerve and hypoglossal nerve were the most often injured nerves, resulting in voice changes, dysphagia, and articulation problems. Among 13 patients whose ICA was exposed, ICA rupture (one intraoperative and one at 1-month postoperation) was reported in two patients, in whom coil embolization was performed. The survival rate was 88.9%, with one patient dying from stroke and pneumonia after ICA rupture, and two due to tumor recurrence, one of whom refused salvage surgery. The final results and a flow chart of the surgical modalities and mucosal status before and after each surgical step are summarized in Table 3 and Fig. 5.
The NSF-V subgroup and NSF-U subgroup comprised 66.7% (n=14) and 33.3% (n=7) of the NSF group, respectively. Age (59.3 vs. 62.7 years, P=0.443), necrosis stage (85.7% vs. 100%, P=0.636), advanced T stage (50% vs. 85.7%, P=0.197), ICA involvement (42.3% vs. 85.7%, P=0.128), and number (1.4 vs. 1.6, P=0.443) and dose (8,651 cGy vs. 10,079 cGy, P=0.799) of RT tended to be higher in the NSF-U subgroup, but no statistically significant differences were found between the two subgroups. The NSF-U subgroup also had a higher tendency for DM and underlying comorbidities (0.0% vs. 28.6% and 14.3% vs. 42.3%, respectively), likewise without a statistically significant difference between the two subgroups (P=0.322 and P=0.322, respectively). Two ICA ruptures and two deaths were reported in the NSF-U subgroup, and one death was reported in the NSF-V subgroup. The median follow-up period was 13.5 months for the NSF-V subgroup and 16 months for the NSF-U subgroup (range, 1–73 months and 5–54 months, respectively). The NSF subgroup analysis is summarized in Table 4.
Even though PRNN severely impacts patients’ quality of life and survival, no standard treatment has been established. Several therapeutic modalities have been introduced, including conservative management using antibiotics and frequent dressing, hyperbaric oxygen therapy, and endoscopic debridement with or without vascularized flap reconstruction. Among these methods, endoscopic debridement is widely used to remove necrotic tissue and replenish fresh tissue, helping to control infection and improve symptoms in the skull base area [7,12]. However, the recovery period is quite long, and necrosis easily relapses; therefore, surgical debridement might need to be repeated [8,11,17]. The exact pathophysiology of PRNN is unclear, but hypoxia, hypovascularity, and hypocellularity from RT are thought to be the key factors in its development and resulting inflammation, sequestration, and erosion of the underlying cortex of the nasopharynx [18,19]. Debridement without flap reconstruction seldom achieves complete healing, and failing to resurface defects of the skull base, dura, or ICA can result in life-threatening complications such as meningitis, ICA blowout, and death [8,10,20]. In fact, ICA involvement has been found to be an independent prognostic factor, and it can increase the risk of death from 41.8%–42.9% to 69.2%–72.7% [7,8,10,11].
Therefore, resurfacing the surgical bed with a vascularized flap and protecting the exposed skull base and ICA are regarded as the most important surgical goals in PRNN treatment. The NSF, pedicled by the posterior septal artery, a branch of the sphenopalatine artery, has been regarded as an effective and reliable reconstruction method for PRNN considering the advancements in image-guided endoscopic skull base surgery [12,15,21]. There have been some challenges in using free flap reconstruction to cover extensive defect sites, after either nasopharyngectomy for recurrent NPC [22,23] or endoscopic debridement for osteoradionecrosis in the head and neck area [24-28]. Although free flap reconstruction is invasive and has some limitations due to the difficult technique, long operation time, and wound problems, it can cover more extensive defects, unlike the NSF, and plays a role in salvage surgery. With its ample size and high vascularity, the ALTFF is a good option for extensive PRNN, especially for cases involving the ICA, which is a known prognostic factor for symptom aggravation, increased disease extent even after surgery, and death [21,25,26]. A recent study by Zou et al. [15] analyzed 72 PRNN patients treated with NSFs. It showed successful outcomes of defect area re-epithelialization (70.8%) and symptom improvement without any surgery-related complications or death, and the 2-year OS rate was 77.9%. They also demonstrated that the viability of NSF reconstruction was a protective factor for re-epithelialization, which could serve as a barrier to protect the ICA.
To our knowledge, outcomes have not been compared according to flap and mucosal status. There is also a lack of research on salvage surgery using a vascularized flap, especially for free flap reconstruction, after surgical failure in PRNN patients. As such, we not only analyzed data corresponding to initial curative intent endoscopic debridement with or without NSF, but also included the clinical courses of salvage operations using vascularized flap reconstruction and compared the clinical outcomes according to final mucosal resurfacing status. Moreover, based on the flap viability of the initial NSF group, we attempted to determine the risk factors for NSF failure in PRNN patients. Our data showed that resurfacing the defect area with an NSF in initial surgery showed better outcomes than debridement only. Most patients in the NSF group achieved healthy mucosal status, and only four patients underwent salvage surgery even in the setting of more ICA involvement, while all six patients in the no-NSF group had to undergo salvage surgery due to recurrent necrosis with recurrent symptoms. Among 10 salvage operations, free flaps were also performed in four patients with ALTFFs (cases 4, 5, 21, 23). Interestingly, although not all of the 10 patients maintained completely healed mucosa after salvage surgery, they also showed good clinical outcomes, with most patients reporting symptom relief. Finally, 21 patients (77.8%) had completely healed mucosa on final presentation, whereas six patients (22.2%) did not. No patients suffered from foul odor, and headaches improved in all, but two patients compared to their initial condition. Among 13 patients whose ICA was involved, two ruptures (case 21: 1-month postoperation, case 24: intraoperative) were reported and rescued by coil embolization. Only three patients died (cases 5, 21, 22), and the survival rate was 88.9%. Our results suggest that endoscopic debridement and mucosal resurfacing with NSF reconstruction are essential for the surgical management of PRNN. Even if the initial surgery is ineffective and the NSF does not cover the whole defect area or becomes necrotized, additional reconstructive resurfacing surgery using another vascularized flap is necessary for better clinical outcomes.
Yang et al. [10] recently reported the clinical outcomes of NPC with PRNN. They demonstrated that osteoradionecrosis, re-irradiation, and ICA involvement affected survival, although only re-irradiation and ICA involvement were independent prognostic factors, with hazard ratios of 1.75 and 1.80, respectively. Additionally, NSF reconstruction was associated with better OS than conservative management. Among 44 patients with NSF in their study, eight flaps (18.2%) did not show favorable re-epithelialization, although an explanation and analysis of risk factors for NSF failure were missing. In our study, when categorizing the NSF group according to flap viability, we found that age, necrosis stage, advanced T stage, ICA involvement, number and dose of RT, and the propensity toward DM and underlying comorbidities tended to be higher in the NSF-U subgroup. Including the variables (e.g., necrosis stage, ICA involvement, and reirradiation) that are already known to be related to the survival of PRNN patients, the factors that we investigated in this study might also play a role in NSF failure due to poor vascularization and perfusion of the underlying nasopharyngeal surface, hindering its healing and mucosalization. Further studies should be conducted with larger cohorts and a sufficient follow-up period, and the pathophysiology should be elucidated to validate these trends.
There were four patients with lower CNP on the initial preoperative examination. Among them, two (cases 23 and 24) experienced partial CNP improvement following salvage surgery, allowing the gastrostomy tube to be removed. During the follow-up period, there were six cases of newly developed CNP, four of which (cases 4, 11, 17, 18; 19.0%) emerged even after final successful flap surgery. One (case 17) of those four cases developed due to a recurrent tumor and another (case 4) after initial surgery without NSF. The other two (cases 11 and 18; 9.5%) in the complete healing group developed new CNP as a consequence of PRNN. In contrast, two patients (cases 1 and 27; 33.3%) with partial or total necrosis newly developed CNP. Therefore, even if the vascularized flap might not fully prevent the development of lower CNP, at least it seems to lessen the likelihood. Nonetheless, we should be cautious that even after successful surgery, necrosis may spread beneath the flap, and CNP may develop and get worse over time. Additionally, although statistically insignificant, a larger proportion of ICA involvement was observed in patients with NSF failure than in the success group (85.7% vs. 42.3%), which implies that if necrotic tissue around the ICA is left in place in order to avoid ICA rupture, there may be a greater chance of flap failure. However, there was a representative example (case 8) of completely healed mucosa and a viable flap with persisting necrotic tissue beneath the mucosal layer on follow-up imaging, but without any symptoms, CNP, or ICA rupture (Fig. 6). Nonetheless, the necrotic bed poses a high risk for flap failure; therefore, it is a dilemma whether to entirely remove the necrotic tissue around the ICA or not. In such instances, total removal of the necrotic tissue following prophylactic ICA embolization or superficial temporal artery (STA) to middle cerebral artery (MCA) bypass surgery may be an excellent option, as described in a recent publication by Cho et al. [16]. In their study, they presented a strategy for managing ICA involvement in which coil embolization was conducted if BOT followed by brain perfusion single-photon emission CT revealed no hypoperfusion, while STA-to-MCA bypass surgery was performed if hypoperfusion was seen. This approach allowed extensive sequestrectomy and successful outcomes, providing valuable insights into future directions of ICA handling in PRNN. Nevertheless, more research on this subject is required.
Despite promising advancements from our previous study and some meaningful results herein, the present study has the limitations of a retrospective analysis, including patient selection bias for each surgical modality since we only performed endoscopic debridement without a vascularized flap as the initial operation and free flap for salvage operation in earlier cases, which changed to the current practice pattern of employing the NSF as our experience and evidence for the utility of the NSF gradually accumulated. Other limitations include the small number of patients who were treated at a single institution and operated on by a single surgeon, and a short follow-up period. Hence, further studies with a longer follow-up period and a larger cohort are required to validate our results.
In conclusion, if mucosal resurfacing was initially not done or improper, the prognosis was not good. Reconstruction using an NSF after endoscopic debridement is an effective and reliable modality in the initial surgical management of PRNN. Even if the initial NSF fails, subsequent resurfacing salvage surgery with a vascularized flap, including free flap reconstruction, should be used to improve clinical outcomes.
▪ In the surgical treatment of post-radiation nasopharyngeal necrosis, resurfacing the nasopharynx with a nasoseptal flap (NSF) after endoscopic debridement showed better outcomes than debridement alone. Therefore, endoscopic debridement with mucosal resurfacing using an NSF in the initial operation is crucial.
▪ Even if the initial mucosal resurfacing with NSF did not cover the entire defect area or had been necrotized, subsequent resurfacing reconstruction surgery provided benefits in terms of symptom relief, quality of life, and survival.
▪ If mucosal resurfacing was initially not done or was done improperly, resurfacing salvage surgery with a vascularized flap, as well as free flap reconstruction, should be implemented for better clinical outcomes.
NotesAUTHOR CONTRIBUTIONS Conceptualization: SDH. Investigation: BS, CHB, MKC. Data curation: HYK, YGJ. Formal analysis: BS, SDH. Methodology: CHB, MKC. Writing–original draft: BS. Writing–review & editing: HYK, YGJ, SDH. SUPPLEMENTARY MATERIALSSupplementary materials can be found via https://doi.org/10.21053/ceo.2022.00465.
Supplementary Table 2.Patients’ demographics and clinical features in salvage surgery Fig. 1.Postoperative mucosal status. (A) Complete healing. (B) Partial healing. (C) Persistent necrosis. 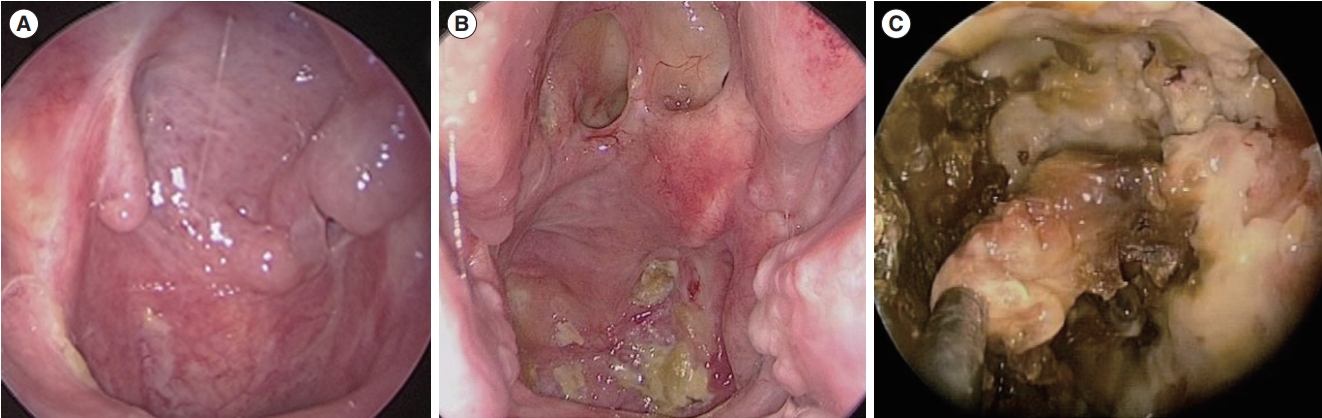
Fig. 2.Stages of necrosis. (A) Early: mucosal necrosis alone. (B) Middle: necrosis involving mucosa, muscle, and tendon. (C) Late: osteoradionecrosis. 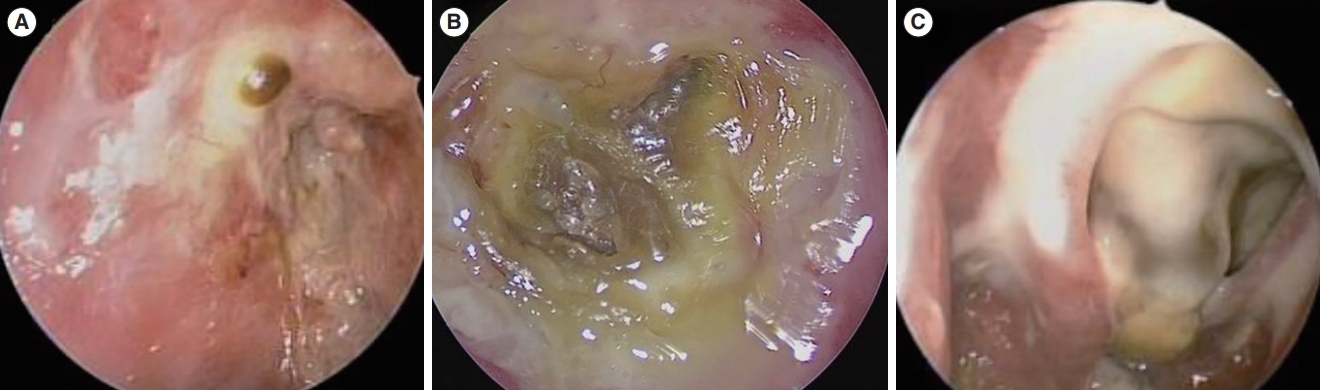
Fig. 3.A representative example of debridement with a nasoseptal flap (NSF) as the initial operation (case 14). (A) Preoperative endoscopic image of radionecrosis. (B) Preoperative magnetic resonance imaging (MRI) shows extensive necrotic lesions on both sides of the nasopharynx, extending to the left internal carotid artery and eroding the clivus. (C) Seventeen months after the operation, a viable NSF with complete mucosal healing was achieved. (D) MRI shows fully viable nasopharyngeal tissue without any remnant necrotic tissue. 
Fig. 4.A case of a salvage operation with an anterolateral thigh free flap (ALTFF) (case 4). (A) Preoperative endoscopic image shows extensive necrosis throughout the entire nasopharynx, nearly extending to the superior margin of the oropharyngeal wall. (B) Intraoperative endoscopic image after debridement; resurfacing with a flap was not performed. (C) A postoperative examination shows extensive necrotic tissue again. (D) Four months after a salvage operation with repetitive debridement and ALTFF; complete healing was achieved. 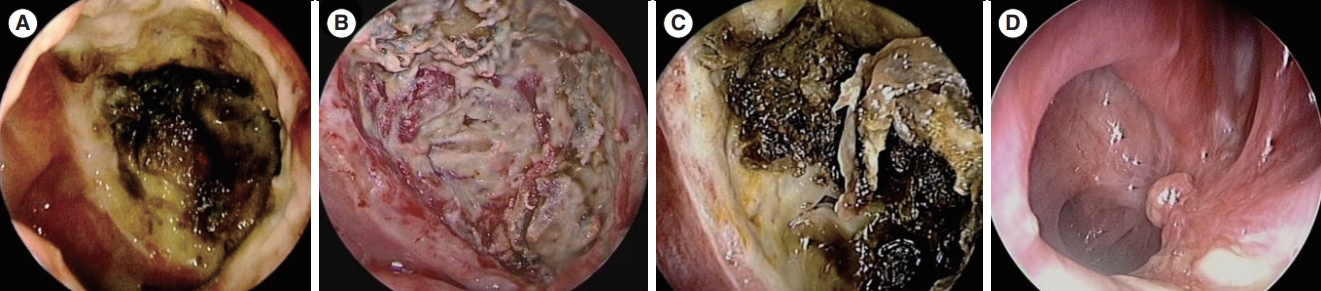
Fig. 5.Flowchart and treatment outcomes. NSF, nasoseptal flap; Op, operation; ALTFF, anterolateral thigh free flap; IT, inferior turbinate; MTF, middle turbinate flap. 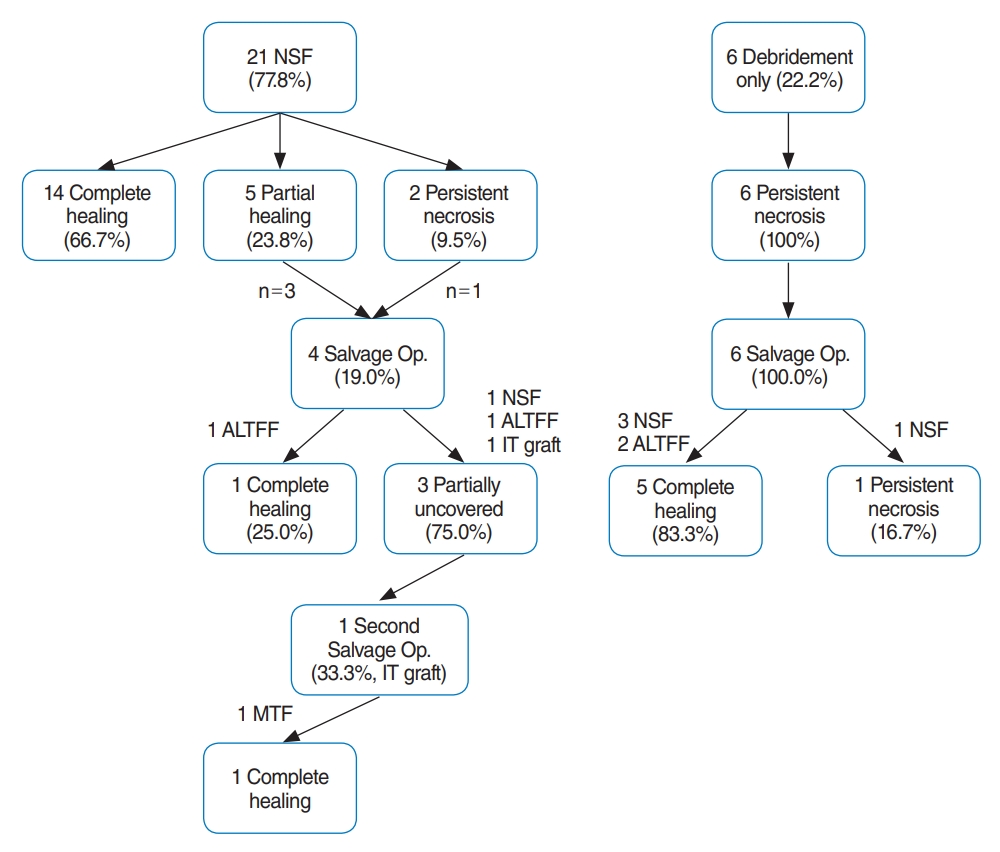
Fig. 6.An example of persistent necrosis under completely healed mucosa in a patient who was free of symptoms (case 8). (A) Preoperative endoscopic image of radionecrosis. (B) Intraoperative endoscopic image after the debridement of necrotic tissue. (C) Fifty-two months after debridement with a nasoseptal flap (NSF); completely healed mucosa with a viable NSF is observed. (D) Preoperative computed tomography (CT) scan shows radionecrosis involving the nasopharyngeal deep tissue near the right internal carotid artery (ICA). (E) CT scan 4 months postoperatively. (F) CT scan 52 months postoperatively; remaining necrotic tissue is seen near the ICA, without any progression compared to the preoperative imaging. 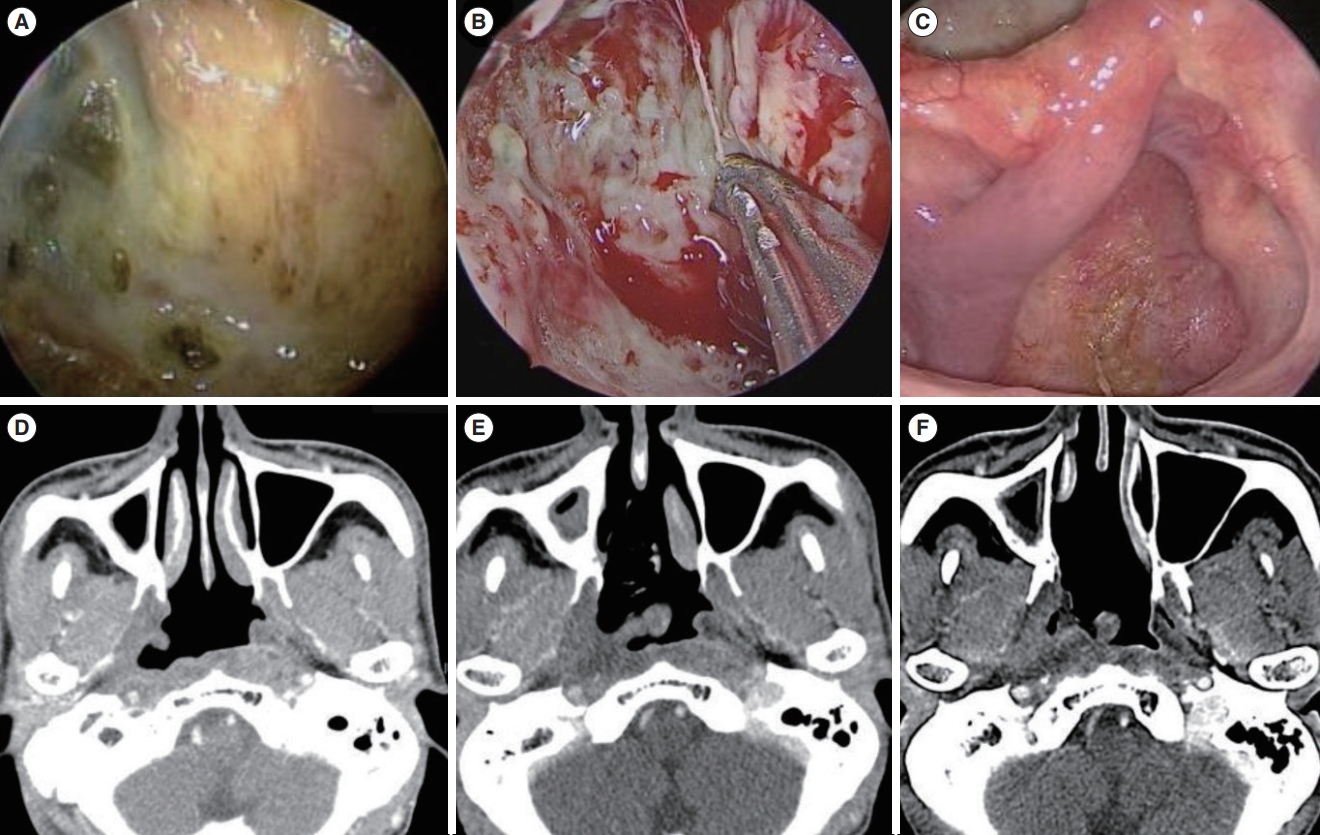
Table 1.Summary of patients’ initial characteristics Table 2.Outcomes of initial surgery in the NSF and no-NSF groups Table 3.Final outcomes Table 4.Subgroup analysis of the NSF group REFERENCES1. Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002 Dec;12(6):421-9.
3. Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui NJ, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001 Mar;19(5):1350-7.
4. Zhang W, Dou H, Lam C, Liu J, Zhou J, Liu Y, et al. Concurrent chemoradiotherapy with or without adjuvant chemotherapy in intermediate and locoregionally advanced nasopharyngeal carcinoma. Tumour Biol. 2013 Jun;34(3):1729-36.
5. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015 Nov;51(17):2587-95.
6. Mao YP, Tang LL, Chen L, Sun Y, Qi ZY, Zhou GQ, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer. 2016 Dec;35(1):103.
7. Huang XM, Zheng YQ, Zhang XM, Mai HQ, Zeng L, Liu X, et al. Diagnosis and management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope. 2006 Sep;116(9):1626-31.
8. Hua YJ, Chen MY, Qian CN, Hong MH, Zhao C, Guo L, et al. Postradiation nasopharyngeal necrosis in the patients with nasopharyngeal carcinoma. Head Neck. 2009 Jun;31(6):807-12.
9. Yu YH, Xia WX, Shi JL, Ma WJ, Li Y, Ye YF, et al. A model to predict the risk of lethal nasopharyngeal necrosis after re-irradiation with intensity-modulated radiotherapy in nasopharyngeal carcinoma patients. Chin J Cancer. 2016 Jun;35(1):59.
10. Yang Q, Zou X, You R, Liu YP, Han Y, Zhang YN, et al. Proposal for a new risk classification system for nasopharyngeal carcinoma patients with post-radiation nasopharyngeal necrosis. Oral Oncol. 2017 Apr;67:83-8.
11. Chen MY, Mai HQ, Sun R, Guo X, Zhao C, Hong MH, et al. Clinical findings and imaging features of 67 nasopharyngeal carcinoma patients with postradiation nasopharyngeal necrosis. Chin J Cancer. 2013 Oct;32(10):533-8.
12. Ryu G, So YK, Seo MY, Park W, Kim HY, Dhong HJ, et al. Using the nasoseptal flap for reconstruction after endoscopic debridement of radionecrosis in nasopharyngeal carcinoma. Am J Rhinol Allergy. 2018 Jan;32(1):61-5.
13. Chen KC, Yen TT, Hsieh YL, Chen HC, Jiang RS, Chen WH, et al. Postirradiated carotid blowout syndrome in patients with nasopharyngeal carcinoma: a case-control study. Head Neck. 2015 Jun;37(6):794-9.
14. Chen MY, Wang SL, Zhu YL, Shen GP, Qiu F, Luo DH, et al. Use of a posterior pedicle nasal septum and floor mucoperiosteum flap to resurface the nasopharynx after endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2012 Oct;34(10):1383-8.
15. Zou X, Wang SL, Liu YP, Liu YL, Zou RH, Zhang YN, et al. A curativeintent endoscopic surgery for postradiation nasopharyngeal necrosis in patients with nasopharyngeal carcinoma. Cancer Commun (Lond). 2018 Dec;38(1):74.
16. Cho SW, Han SY, Song Y, Kim JW, Kim HJ, Kim DY, et al. Aggressive treatment including endonasal surgical sequestrectomy with vascularized nasoseptal flap can improve outcomes of skull base osteoradionecrosis. J Neurol Surg B Skull Base. 2021 Jan;83(Suppl 2):e15-23.
17. Hua YJ, Chen MY, Hong MH, Zhao C, Guo L, Han F, et al. Short-term efficacy of endoscopy-guided debridement on radiation-related nasopharyngeal necrosis in 20 nasopharyngeal carcinoma patients after radiotherapy. Ai Zheng. 2008 Jul;27(7):729-33.
18. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983 May;41(5):283-8.
19. Lou PJ, Chen WP, Tai CC. Delayed irradiation effects on nasal epithelium in patients with nasopharyngeal carcinoma: an ultrastructural study. Ann Otol Rhinol Laryngol. 1999 May;108(5):474-80.
21. Adel M, Chang KP. Using a nasoseptal flap for the reconstruction of osteoradionecrosis in nasopharyngeal carcinoma: a case report. J Otolaryngol Head Neck Surg. 2016 Apr;45:27.
22. Khoo ML, Soo KC, Gullane PJ, Neligan PC, Hong SW, Lee JC, et al. Resurfacing of the nasopharynx after nasopharyngectomy using a free radial forearm flap. Head Neck. 2001 Oct;23(10):916-22.
23. Baek CH, Park W, Choi N, Gu S, Sohn I, Chung MK. Free flap outcome of salvage surgery compared to primary surgery for head and neck defects: a propensity score analysis. Oral Oncol. 2016 Nov;62:85-9.
24. Gordin EA, Ducic Y. Microvascular free tissue reconstruction in the patient with multiple courses of radiation. Laryngoscope. 2014 Oct;124(10):2252-6.
25. Choi NY, Kim HJ, Baek CH. Surgical management of extensive osteoradionecrosis in nasopharyngeal carcinoma patients with the maxillary swing approach and free muscular flaps. Clin Otolaryngol. 2017 Oct;42(5):1100-4.
26. Vlantis AC, Wong EW, Chiu TW, Chan JY. Vastus lateralis muscle free flap for skull base osteoradionecrosis in nasopharyngeal carcinoma. J Neurol Surg B Skull Base. 2018 Aug;79(4):349-52.
|
|
|||||||||||||||||||||||||||||||||||||||||||||







