 |
 |
- Search
AbstractConventional surgery through a transcervical incision is indicated for the treatment of certain tumors in the head and neck. However, this method can cause multiple problems, including scarring and cosmetic concerns. The endoscope-assisted hairline approach, which serves as an alternative to conventional surgical procedures, is gaining popularity due to its excellent cosmetic and functional outcomes. However, given the anatomical complexity involved, the endoscope-assisted hairline technique is not frequently employed in head and neck surgery. The evolution of the hairline surgical approach has been influenced by changes in disease conditions and recent advances in surgical tools. This review article discusses the use of endoscope-assisted hairline approaches in the resection of head and neck masses, focusing on the surgical procedure and postoperative clinical outcomes.
Endoscopic surgery has gained popularity because it is relatively minimally invasive and offers cosmetic benefits. Conventional transcutaneous excision is a well-established surgical technique for removing lesions in the head and neck. However, the external transcervical percutaneous incision inevitably leaves scarring. Even when the incision is strategically placed along skin creases and expression lines, the scars can become hypertrophic or develop into keloids. Scars that result from head and neck lesions can have psychological and social impacts on patients. Therefore, the cosmetic outcomes of surgery are important to consider [1-3].
Numerous efforts have been undertaken to conceal or minimize the incision scars associated with head and neck surgery. Since the 1980s, minimally invasive surgery has been popular across all surgical specialties. Traditional surgical methods in thoracic and abdominal surgery have been largely replaced by endoscopic surgery. In 1996, Gagner was the first to describe endoscopic subtotal parathyroidectomy in head and neck lesions, using constant gas insufflation for hyperparathyroidism. This technique results in a smaller neck scar [4-9].
In the realm of minimally invasive surgery, new surgical techniques are constantly being developed. These methods are characterized by their low invasiveness and favorable cosmetic outcomes [10-12]. One such technique is the hairline approach, in which incisions are made in such a way that they are not visible, providing a cosmetic advantage by eliminating noticeable scarring (Fig. 1). Notably, despite the terminological similarity, the hairline approach is distinct from the retroauricular hairline incision.
While the hairline approach is typically restricted to benign lesions, current initiatives aim to broaden its application to malignant head and neck lesions. From a cosmetic acceptance standpoint, this approach is significantly superior to the percutaneous transcervical approach, without a significant increase in complications or hospitalization (Fig. 2) [13-19].
However, given the intricate nature of head and neck anatomy, the widespread use of such surgical methods is yet to be seen. Consequently, new strategies are being devised to conceal or eliminate incision scars resulting from the resection of head and neck lesions. Between 2012 and 2018, we employed the hairline approach in the excision of head and neck masses among 65 patients. We recorded their age, sex, tumor size, incision length, pathological findings, complications, and satisfaction scores (Table 1). This review provides an overview of the advancements made over these years. All photos of the figures used in the paper were agreed upon in advance.
A palpable lesion in the submandibular gland is typically indicative of a genuine salivary gland tumor. Conditions such as salivary gland neoplasm, chronic sialadenitis with or without sialolithiasis, plunging ranula, tuberculous sialadenitis, and drooling often necessitate the excision of the submandibular gland. In this context, we highlight the submandibular gland as a successful area for mass removal in this region [20].
Approximately 7%–11% of all salivary gland tumors originate from the submandibular gland, with 30%–54% of these being malignant [21]. Such tumors typically manifest as swollen masses in the submandibular region. The initial diagnosis of benign lesions is usually made in patients who are at least 50 years old, while malignant lesions are typically first diagnosed in patients aged 60 years or older. The majority of submandibular gland tumors present as painless, firm masses, best detected through bimanual palpation [22].
The only effective treatment for submandibular gland tumors is surgical resection. A number of surgical techniques have been developed for the removal of the submandibular glands, including intraoral, transoral, and submental resections [23-25]. The conventional method, which is well-established, involves a percutaneous transcervical approach via a standard submental incision. However, this procedure can occasionally lead to an unsatisfactory visible scar and may also result in neurological damage to the facial, hypoglossal, and lingual nerves [26,27].
The hairline approach outlined in this text allows surgeons to easily identify the submandibular ganglion, lingual nerve, hypoglossal nerve, and submandibular gland. While this approach may offer a restricted surgical field, the use of a magnified endoscope compensates for this by providing a clear view. This enables the capsule of a specific lesion to be clearly identified and preserved [13,28,29].
Patients who underwent surgery using the hairline approach reported significantly higher satisfaction than those in the conventional group. Furthermore, no significant increases were noted in either complications or the length of hospital stay.
Prior to surgery, the hair was shaved up to 3 cm above the hairline on the affected side. The hairline was then indicated with a surgical marking pen to denote the location of the incision and the working space. The surgical team consisted of an operator and an assistant. The assistant was responsible for holding the endoscope, which allowed the operator to utilize both hands throughout the procedure.
Each patient was positioned supine under general anesthesia, with shoulder rolls placed under both shoulders. The head was turned to face the side opposite the lesion, and the neck was extended. To prevent slipping from the surgical bed, the head was securely fixed. The skin preparation and draping methods used were identical to those employed for conventional submandibular gland surgery. An incision measuring between 50 and 70 mm was made on the scalp, 1 cm behind the hairline. Subsequently, the scalp flap was elevated using Metzenbaum scissors and two-prong retractors. During this process, care was taken to protect the great auricular nerve and to avoid damage to the hair follicles.
Using a monopolar coagulator, the subplatysmal skin flap was elevated just superior to the sternocleidomastoid (SCM) muscle, with the head oriented in an anterior and inferior direction. The submandibular gland was partially exposed, and the scalp flap was raised to establish a working space with sufficient height for the insertion of Sofield retractors into the surgical field. The incision plane between the capsule of the submandibular gland and the surrounding tissues could be clearly identified.
The operator held DeBakey forceps in the left hand to secure and adjust the position of the mass, while a harmonic scalpel was held in the right hand to carry out the resection. The facial artery, facial vein, and Wharton duct were ligated using the harmonic scalpel. The magnified view provided by the endoscope facilitated the easy identification and preservation of the lingual and hypoglossal nerves (Fig. 3). The submandibular gland, inclusive of the intraglandular masses, was subsequently excised. Following the removal of the gland, a Penrose drain was inserted through the hairline incision line. The incision was then tightly closed using interrupted sutures [13].
The hairline approach, as described above, was performed in 20 patients, who were compared with another 20 individuals who underwent conventional transcutaneous surgery. The evaluated parameters included age, tumor size, incision length, amount of drainage, and duration of the operation. Among these parameters, only the operation duration varied significantly between the two groups, with a longer duration observed in patients who underwent the hairline approach. One patient (5%) in the hairline approach group experienced temporary facial nerve palsy. However, this patient was determined to have Bell palsy, which affected the entire facial nerve, and recovered after 2 months. Additionally, two patients (10%) in the conventional group experienced temporary facial nerve palsy, but they similarly recovered within 2 months.
Parotid gland tumors are the most common type of salivary gland tumors, accounting for about 80%. In turn, approximately 80% of these tumors arise in the superficial lobe, predominantly in the infra-auricular area [20,30]. The preauricular location is less frequently observed. Parotid tumors are relatively rare, with an annual occurrence of 17.6 per million, and 77% are benign. The most common subtype is pleomorphic adenoma [31].
Parotidectomy, a well-established surgical technique, is the only effective treatment for parotid tumors. The extent of surgery determines the type of parotidectomy performed, which can be extracapsular dissection, partial parotidectomy, superficial parotidectomy, or total parotidectomy [32-34]. To provide better exposure and enable delicate dissection to prevent facial nerve injury, these techniques are performed using either a Blair incision or a facelift incision. The most frequent complications following these procedures are facial nerve paresis and paralysis [35].
The hairline technique outlined herein provides surgeons with a straightforward method for locating tumors and the branches of the facial nerve. Furthermore, the resulting incision scars are not visible; even when a hypertrophic scar forms, it remains hidden beneath the hair [17,36]. The hairline approach in parotid surgery offers an advantage in that it allows for the safe dissection of tumors without causing injury to the facial nerve. This is enabled through the use of an endoscope, which provides magnification, and a neurological monitoring system.
The patients were placed in the supine position, with a pillow under the shoulder, while under general anesthesia. The neck was extended, and the head was rotated to the side opposite the lesion. Prior to draping, the locations of the mass, incision line, and working space were outlined using a marking pen. Subsequently, the skin preparation and draping procedures used in traditional parotid surgery were implemented. An incision of approximately 50–70 mm was made over the postero-inferior auricular scalp, 1 cm posterior to the hairline. The scalp flap was then elevated using Metzenbaum scissors and right-angle retractors.
The superficial layer of the parotid gland and the SCM muscle were exposed through anterior dissection of the scalp flap. Further dissection was carried out between the posterior section of the parotid gland and the SCM muscle. During this dissection, the peripheral branch of the facial nerve was identified using an endoscope and was gently retracted away from the tumor. Given that facial nerve damage is the most preventable adverse effect of this procedure, we employed a meticulous surgical technique. We utilized the NIM-Response 2.0 nerve-monitoring system (Xomed) to confirm the sections of the facial nerve, including peripheral branches. The magnified endoscopic view allowed us to clearly identify the branches of the nerve. Subsequently, we performed careful dissection of the tumor to avoid damage to the facial nerve.
The tumor was identified after successful dissection and subsequently extracted through the surgical wound. Wound irrigation and bleeding control were then performed (Fig. 4). After the insertion of a Hemovac drain through the hairline incision line, the incision was closed using interrupted sutures [17]. The described approach was performed in 18 surgical procedures. The median operation duration was 82.5±18.5 minutes, and the median incision length was 55±0.62 mm. All surgical wounds healed completely, with no complications such as skin flap necrosis, color changes, or hematomas. One patient (6%) experienced transient facial nerve palsy (House-Brackmann grade II) but recovered within 1 month.
Head and neck masses are typically identified through physical examination. Given the complex anatomical structures and important functions associated with the head and neck region, it is essential to preserve both the morphology and function in optimizing the effectiveness of surgical treatment [37-39]. Branchial cleft cyst presents as a unilateral swelling of the soft tissue, located on the lateral aspect of the neck and anterior to the SCM muscle. Approximately 95% of branchial anomalies are second branchial cleft abnormalities. The second branchial cleft is associated with the submandibular gland or is found in the anterior triangle of the neck [40,41].
The treatment of a branchial cleft cyst necessitates a profound understanding of the cyst’s embryogenesis. In the initial 6 to 7 weeks of fetal life, the fetal branchial arches form pockets within the ectodermal epithelium due to incomplete closure or obliteration. These pockets typically become filled during fetal development. However, if this filling process is inadequate, the pockets can evolve into cysts, sinuses, and fistulas [42].
For benign lesions in the head and neck region, surgical resection is often required. The conventional transcervical approach is a well-established surgical technique used for this purpose. However, this procedure can lead to scarring. While some scars may heal well and align with the neck’s natural wrinkles, others may develop into hypertrophic scars. Scarring in the head and neck regiJon can substantially impact social interactions. Therefore, surgeons should consider the potential postoperative cosmetic outcomes when performing operations in these areas [40].
Following general anesthesia by oral intubation, the patient was positioned supine with the head rotated away from the side of the lesion. A computed tomography examination was utilized to enable the operators to evaluate the lesion in advance. Prior to draping, the location of the mass, the incision line, and the working space were indicated with a marking pen. The procedures for skin preparation and draping were consistent with those employed for transcutaneous neck surgery. An incision of 50–70 mm was carefully made over the scalp, approximately 1 cm behind the hairline, avoiding damage to the hair follicles. Subsequently, the scalp flap was meticulously elevated to preserve the great auricular nerve, then was lifted to create a tunnel beneath the skin.
The scalp flap was retracted using Sofield retractors (Zimmer Biomet). This retraction of the flap not only provides a clear view of the surgical field, but also reduces bleeding. Furthermore, it lessens the risk of damage to the vessels and nerves. Following the elevation of the flap, the endoscope was introduced into the operative field. Using one hand, the surgical assistant elevated the scalp flap to ensure adequate working space for the operation. The other hand was employed to adjust the endoscope’s position, allowing the operator to achieve an accurate view. This arrangement facilitated the operator’s use of both hands during the procedure.
The mass was entirely removed by performing a dissection through the avascular plane, which allowed for the separation of the mass from the surrounding tissue. To minimize the risk of mass rupture, it was crucial to preserve the capsule of the lesion. The mass was then identified and extracted through the surgical incision. Following this, wound irrigation was carried out and bleeding was controlled (Fig. 5). A silastic drain was inserted through the wound, and the incision was tightly closed using interrupted sutures (Fig. 6) [18].
We employed this technique in 27 procedures and compared various parameters, including age, tumor size, incision length, amount of drainage, and duration of the operation, with those of 28 patients who underwent traditional transcutaneous surgery. The only significant difference between the two groups was the duration of the operation, which was longer for patients who underwent surgery using the hairline approach. In each group, one patient experienced temporary damage to the great auricular nerve, but both recovered within 2 months. One patient who had undergone transcutaneous surgery experienced facial nerve palsy affecting the buccal branch; however, the symptoms disappeared within 3 months.
The surgical technique outlined herein represents the most recent method for removing a head and neck mass via the hairline approach, and numerous studies are currently underway on this topic [43]. The hairline approach offers several advantages, the foremost being superior cosmetic outcomes. When planning surgery, the potential for postoperative scarring is a crucial consideration. In head and neck surgery, scars are inevitably situated on the neck, a region integral to social interactions. The hairline approach has been shown to yield significantly higher satisfaction levels compared to the transcutaneous approach. The primary advantage of the hairline approach is its excellent cosmetic outcomes, which contribute to the emotional satisfaction of patients [44].
Minimally invasive surgical techniques in the head and neck region have seen consistent advances. A variety of thyroidectomy methods have been explored, including transaxillary, transoral, submental, and retroauricular approaches, all of which are known to produce good cosmetic outcomes. However, for other head and neck masses, apart from the retroauricular approach, alternative methods are seldom employed due to the considerable distance between the incision and the mass. Both the retroauricular hairline incision and the hairline approach produce good cosmetic outcomes. However, the hairline approach offers additional benefits: the endoscope provides a magnified and clear view, enabling precise dissection and preservation of delicate structures, including specific nerves. Furthermore, the use of an endoscope allows a smaller working space, which is beneficial for postoperative healing.
Traditional parotidectomy is typically conducted through an approximately 10-cm-long incision. The incision line can substantially impact postoperative aesthetics, particularly in patients prone to scar formation. Consequently, numerous efforts have been made to obscure the incision scar. Various incision types have been employed, including preauricular and postauricular incisions. In a similar vein, the endoscope-assisted hairline approach allows for the incision scar to be hidden beneath regrown hair, resulting in improved cosmetic outcomes.
The hairline approach to the submandibular gland allows the surgeon to easily identify the submandibular ganglion, lingual nerve, hypoglossal nerve, and submandibular gland. This method also allows for the safe preservation of the marginal mandibular nerve through its direct identification and upward retraction following the ligation of the facial vein. Furthermore, the harmonic scalpel operates by generating heat through vibration, rather than electricity, making it less damaging to the nerve [45].
The most common complication anticipated during a parotidectomy is facial nerve paresis or paralysis. However, endoscopeassisted parotidectomy offers an advantage in this regard via nerve-monitoring and endoscope systems. The magnification provided by the endoscope facilitates the clear identification of the facial nerve branches, while the nerve-monitoring system enables the differentiation of these branches. Once the facial nerve is identified, the surgeon simply needs to gently retract it away from the tumor [14].
The outcomes following the hairline approach are generally more favorable than those achieved with traditional techniques. The satisfaction score, a measure of the patient’s subjective contentment with the postoperative wound, has been consistently high among hairline incision groups. This score has demonstrated a statistically significant difference relative to the conventional group. This suggests that, to a significant degree, patients who underwent the hairline approach were satisfied due to the concealment of scarring [13,17,18].
The primary drawback of endoscopic excision is its extended operation time. The hairline approach takes longer than the percutaneous transcervical approach due to the time required to elevate the scalp flap and establish the necessary workspace for the endoscope-assisted method. Nevertheless, all procedures utilizing the hairline approach were concluded within 120 minutes. Despite the longer operation time being a disadvantage, it may be deemed acceptable when considering the cosmetic results. In patients with short hair, any residual scars are concealed within the hairline, making them difficult to see. Temporary numbness in the earlobe following procedures using the hairline approach may be attributed to damage to the great auricular nerve due to traction. Of the 65 patients, four experienced temporary numbness in the earlobe, which resolved within 3 months.
Compared to traditional open surgery, endoscopic surgery may present a more confined workspace. In contrast, conventional open surgery can effectively expose the entire surgical field, offering a broad view of the operation. Nevertheless, endoscopic surgery has an advantage in that the magnified view provided by the endoscope results in a clearer visual, leading to a more accurate surgical procedure. Ongoing advancements in surgical procedures underscore the importance of minimally invasive techniques. Gagner pioneered the first endoscopic subtotal parathyroidectomy in 1997, and since then, a variety of remote access techniques have been employed in head and neck surgery. Among these, the hairline approach has been the subject of active research, utilizing the most recent technology. While this approach presents technical challenges, it offers superior cosmetic results without leading to significant complications.
Continuous innovations and enhancements are being made in the realm of endoscope-assisted hairline approaches. Numerous opportunities for improvement exist across various areas, one of which is the skill level of the operator. In the future, the potential exists for collaboration with those in the field of minimally invasive surgery. In that area, a range of techniques are under development, including the incorporation of the Da Vinci robotic system. Robotic surgical systems afford the surgeon a magnified, three-dimensional view of the surgical field from multiple perspectives. By integrating these endoscope-assisted hairline approaches with the precision of robotic surgery, superior outcomes can be anticipated.
This review presents the endoscope-assisted hairline approach for the treatment of head and neck masses. This method is strongly advocated as an alternative surgical option for procedures involving the head and neck, with the anticipation that it will ultimately supersede traditional skin incisions.
▪ The endoscope-assisted hairline approach is becoming a favored option for many surgical techniques in the head and neck region.
▪ This approach can effectively yield superior cosmetic outcomes.
▪ The endoscope-assisted hairline approach is a viable surgical alternative to the traditional transcutaneous approach.
CONFLICT OF INTERESTSeung Hoon Woo is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported. NotesAUTHOR CONTRIBUTIONS Conceptualization: SHW. Data curation: SHW. Formal analysis: SHW. Funding acquisition: SHW. Methodology: SHW. Project administration: SHW. Supervision: SHW. Validation: SHW. Visualization: MSS. Writing–original draft: MSS. Writing–review & editing: MSS. ACKNOWLEDGMENTSThis work was supported by the Dankook Institute of Medicine and Optics in 2023. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (RS-2023-00247651 and NRF-2020R1A6A1A03043283), Leading Foreign Research Institute Recruitment Program through NRF funded by the Ministry of Science and ICT (NRF-2023K1A4A3A02057280), Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare (HI20C2088), Korea Medical Device Development Fund grant funded by the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety (KMDF_PR_20200901_0027-03), Republic of Korea. The present research was supported by the research fund of Dankook University Research and Business Development Foundation in 2023.
Informed consent for the use of preoperative and postoperative facial photographs was obtained from all patients with a full explanation.
Fig. 1.A 2×2-cm submandibular mass was excised using the hairline approach. (A) Markings were made preoperatively. (B) A 50-mm incision was made on the scalp, 1 cm behind the hairline. (C) The scalp flap was elevated to provide adequate height for the insertion of Sofield retractors into the surgical field. 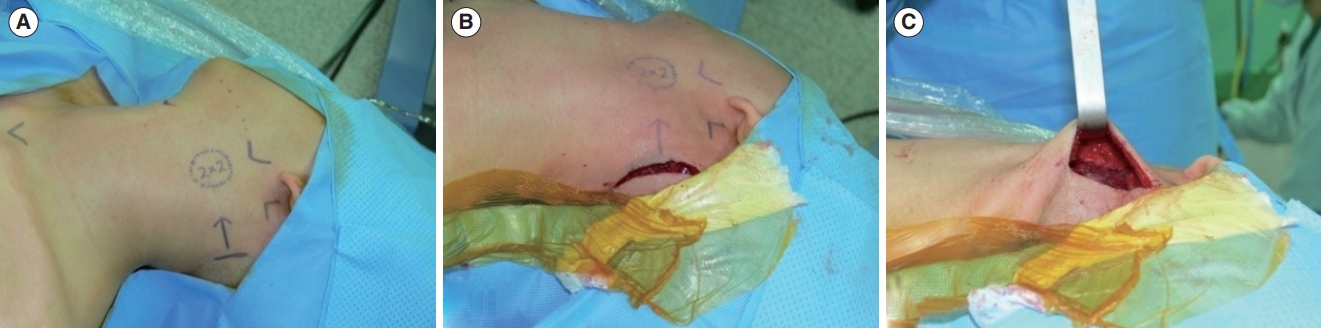
Fig. 2.The hairline approach offers a cosmetic advantage by eliminating visible scars. (A) The surgical wound was positioned at the edge of the hairline. (B) Three months after surgery, the scar was concealed by the regrowth of hair. (C) A male patient 3 months after surgery. Even in patients with short hair, scars were seldom noticeable because they were situated along the hairline. 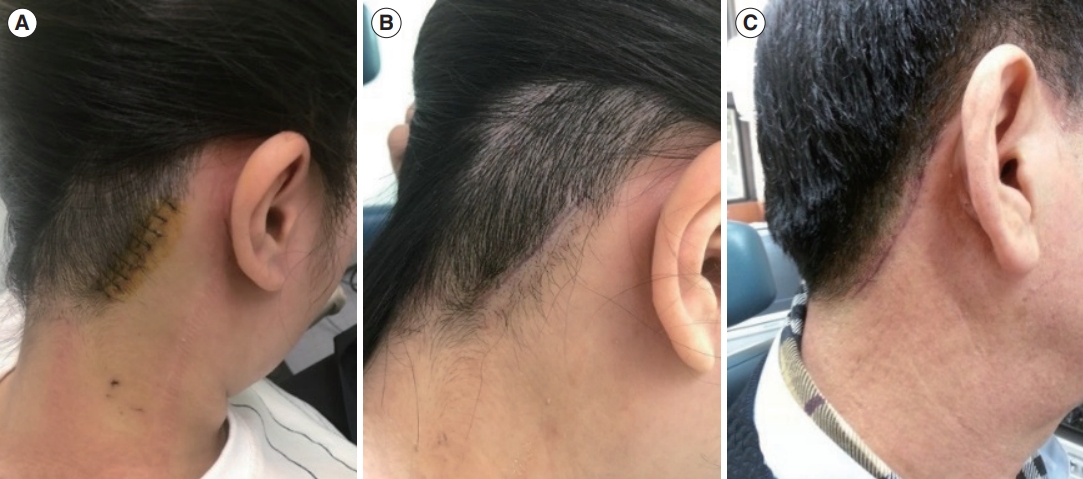
Fig. 3.An endoscopic view of submandibular gland (SMG) mass excision performed via a hairline incision. (A) The subplatysmal skin flap was elevated to establish an adequate working space. (B) Endoscopic guidance was utilized to identify any mass-like lesion. (C, D) Following meticulous dissection in the anterior and inferior direction, the SMG was identified. (E) The facial artery was easily identified with the aid of the endoscope and was preserved. (F) The SMG was subsequently excised and extracted through the surgical wound. 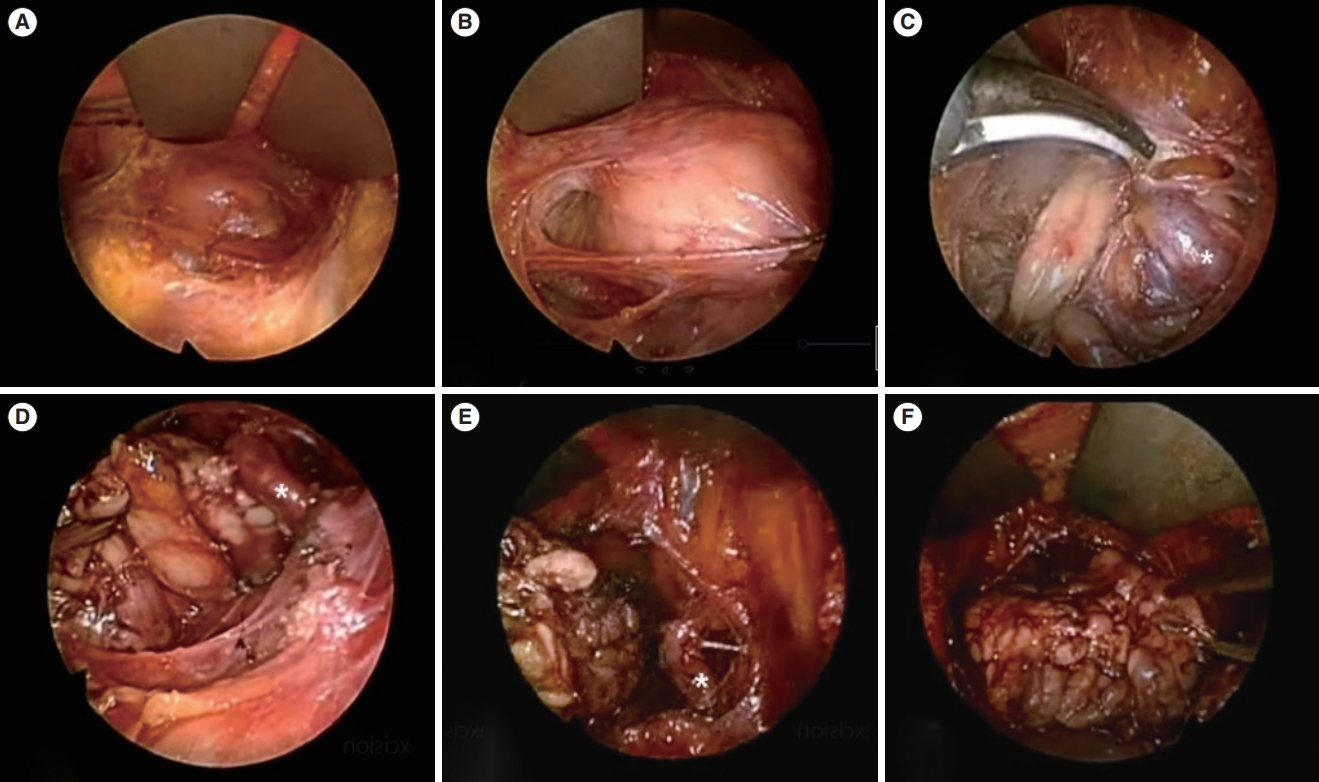
Fig. 4.A parotid mass was excised using the hairline approach. (A) The scalp flap was elevated following anterior dissection. (B, C) Anterior dissection was performed to reveal the sternocleidomastoid muscle and superficial parotid. (D, E) The parotid gland was surgically removed and extracted through the surgical wound. 
Fig. 5.A neck mass was removed using the hairline approach. (A) An incision was created on the scalp, approximately 1 cm posterior to the hairline. (B) The scalp flap was elevated and extracted. (C, D) Meticulous dissection was performed, revealing the lesion. (E, F) The mass was subsequently excised and removed through the surgical wound. After histopathological examination, the mass was identified as a branchial cleft cyst. 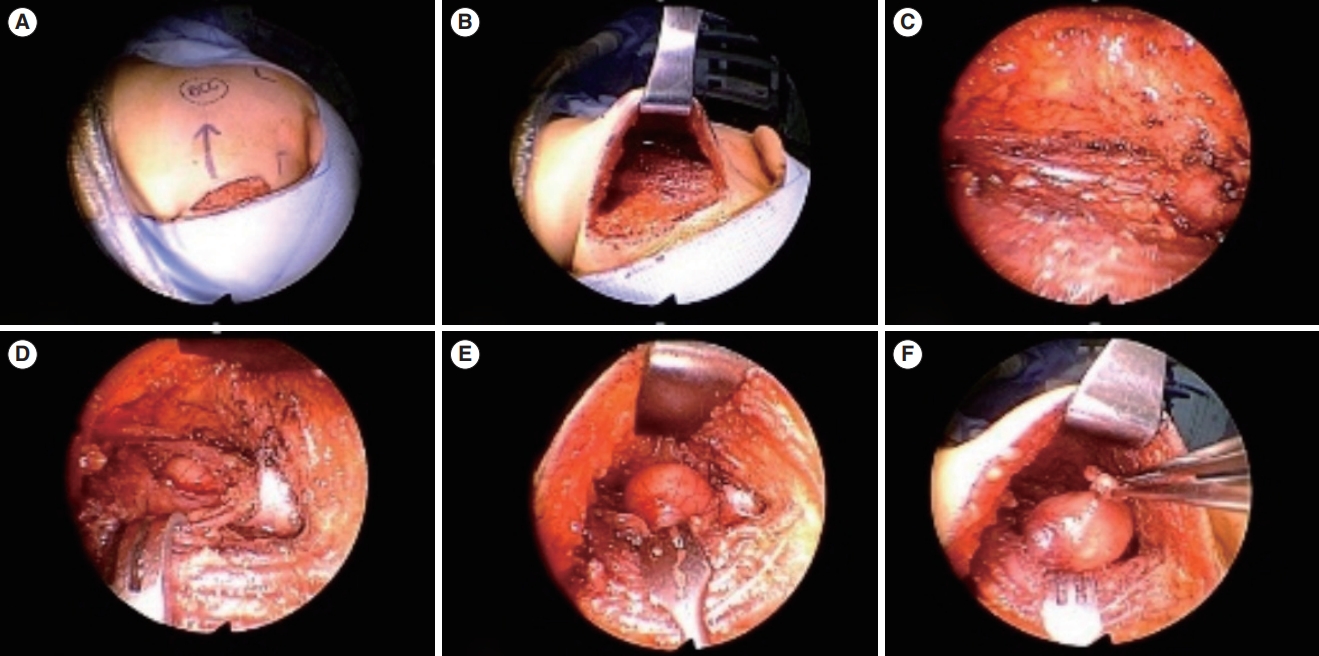
Fig. 6.A cystic mass located in the lateral neck was excised. (A, B) An incision was made along the hairline, and the flap was elevated. (C, D) Endoscopic view of the surgical procedure. The mass was identified using a magnified view and clearly resected. (E) After tumor removal, the great auricular nerve (indicated by the arrow) was preserved. (F) At 1 week after surgery, the scar could be noted on the hairline and will not be visible after the hair grows. 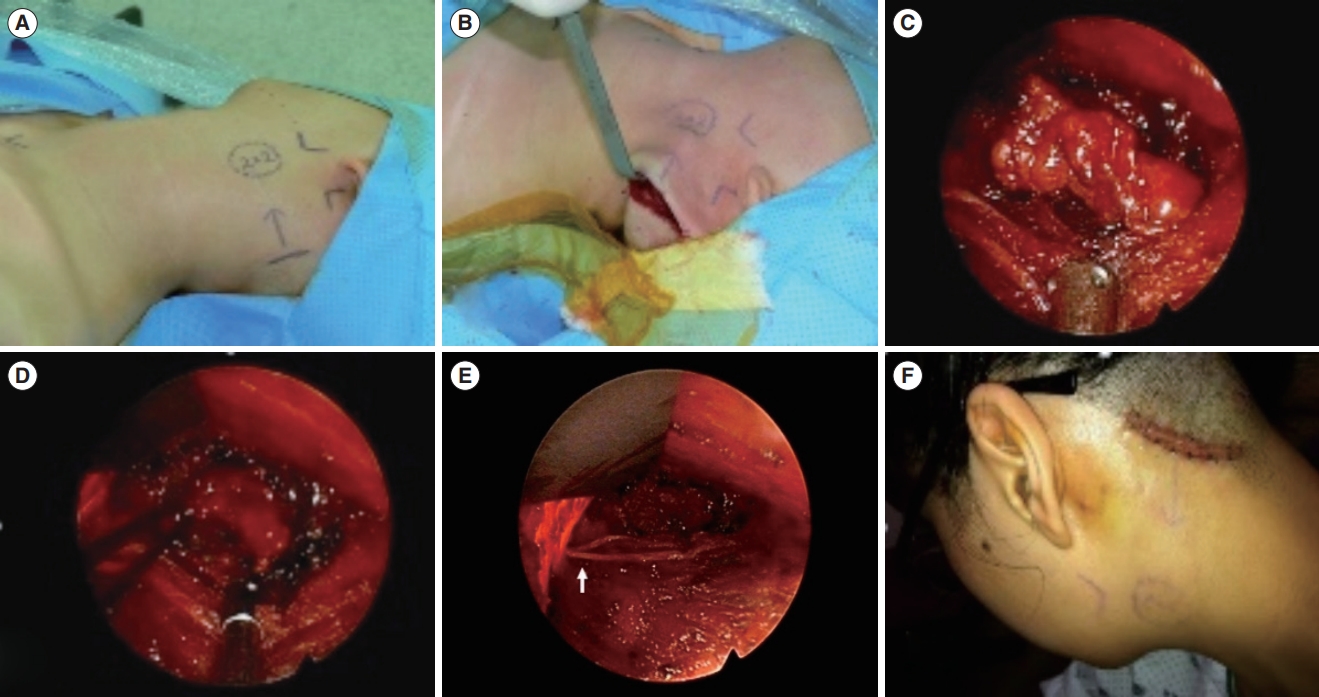
Table 1.Demographics, tumor characteristics and details of the submandibular gland, parotid gland, and lateral neck mass group
REFERENCES1. Rumsey N, Clarke A, White P. Exploring the psychosocial concerns of outpatients with disfiguring conditions. J Wound Care. 2003 Jul;12(7):247-52.
2. Lee HS, Lee D, Koo YC, Shin HA, Koh YW, Choi EC. Endoscopic resection of upper neck masses via retroauricular approach is feasible with excellent cosmetic outcomes. J Oral Maxillofac Surg. 2013 Mar;71(3):520-7.
3. Kim J, Lee YI, Lee JH, Oh SH, Lee SE, Kim YK. Successful treatment of post-operative keloid with combined cryotherapy and ablative fractional CO2 laser. Med Laser. 2020 Jun;9(1):58-61.
4. Gagner M, Inabnet WB. Endoscopic thyroidectomy for solitary thyroid nodules. Thyroid. 2001 Feb;11(2):161-3.
5. Ikeda Y, Takami H, Sasaki Y, Kan S, Niimi M. Endoscopic neck surgery by the axillary approach. J Am Coll Surg. 2000 Sep;191(3):336-40.
6. Ikeda Y, Takami H, Sasaki Y, Takayama J, Niimi M, Kan S. Comparative study of thyroidectomies: endoscopic surgery versus conventional open surgery. Surg Endosc. 2002 Dec;16(12):1741-5.
7. Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996 Jun;83(6):875.
8. Choi WJ, Park ES, Tak MS, Kang SG. Combination treatment of Nd: YAG picosecond-domain laser and fractional CO2 laser for contracted neck scar with hyperpigmentation. Med Lasers. 2021 Feb;10(1):52-4.
10. Miccoli P, Bendinelli C, Berti P, Vignali E, Pinchera A, Marcocci C. Video-assisted versus conventional parathyroidectomy in primary hyperparathyroidism: a prospective randomized study. Surgery. 1999 Dec;126(6):1117-21.
11. Ohgami M, Ishii S, Arisawa Y, Ohmori T, Noga K, Furukawa T, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech. 2000 Feb;10(1):1-4.
12. Park KN, Cho SH, Lee SW. Nationwide multicenter survey for current status of endoscopic thyroidectomy in Korea. Clin Exp Otorhinolaryngol. 2015 Jun;8(2):149-54.
13. Woo SH, Park JJ, Kwon M, Kim JP. “Hidden scar” submandibular gland excision using an endoscope-assisted hairline approach. Oral Oncol. 2017 Feb;65:83-8.
14. Woo SH. Endoscope-assisted intraoral removal of the thyroid isthmus mass using a frenotomy incision. J Laparoendosc Adv Surg Tech A. 2013 Sep;23(9):787-90.
15. Woo SH. Endoscopic-assisted total thyroidectomy via lateral keloid scar incision. Clin Exp Otorhinolaryngol. 2014 Dec;7(4):338-41.
16. Woo SH, Jeong HS, Kim JP, Park JJ, Baek CH. Endoscope-assisted intraoral removal of ectopic thyroid tissue using a frenotomy incision. Thyroid. 2013 May;23(5):605-8.
17. Woo SH, Kim JP, Baek CH. Endoscope-assisted extracapsular dissection of benign parotid tumors using hairline incision. Head Neck. 2016 Mar;38(3):375-9.
18. Kim JP, Park JJ, Woo SH. Endoscope-assisted hairline approach for resecting maxillofacial masses. Int J Oral Maxillofac Surg. 2020 Mar;49(3):310-6.
19. Tae K, Ji YB, Song CM, Ryu J. Robotic and endoscopic thyroid surgery: evolution and advances. Clin Exp Otorhinolaryngol. 2019 Feb;12(1):1-11.
20. Eneroth CM. Salivary gland tumors in the parotid gland, submandibular gland, and the palate region. Cancer. 1971 Jun;27(6):1415-8.
21. Aro K, Tarkkanen J, Saat R, Saarilahti K, Makitie A, Atula T. Submandibular gland cancer: specific features and treatment considerations. Head Neck. 2018 Jan;40(1):154-62.
22. Weber RS, Byers RM, Petit B, Wolf P, Ang K, Luna M. Submandibular gland tumors. Adverse histologic factors and therapeutic implications. Arch Otolaryngol Head Neck Surg. 1990 Sep;116(9):1055-60.
23. Preuss SF, Klussmann JP, Wittekindt C, Drebber U, Beutner D, Guntinas-Lichius O. Submandibular gland excision: 15 years of experience. J Oral Maxillofac Surg. 2007 May;65(5):953-7.
24. Singer DP, Sullivan PK. Submandibular gland I: an anatomic evaluation and surgical approach to submandibular gland resection for facial rejuvenation. Plast Reconstr Surg. 2003 Sep;112(4):1150-4.
25. Laskawi R, Ellies M, Arglebe C, Schott A. Surgical management of benign tumors of the submandibular gland: a follow-up study. J Oral Maxillofac Surg. 1995 May;53(5):506-8.
26. Conley J, Myers E, Cole R. Analysis of 115 patients with tumors of the submandibular gland. Ann Otol Rhinol Laryngol. 1972 Jun;81(3):323-30.
27. Munir N, Bradley PJ. Diagnosis and management of neoplastic lesions of the submandibular triangle. Oral Oncol. 2008 Mar;44(3):251-60.
28. Chen MK, Su CC, Tsai YL, Chang CC. Minimally invasive endoscopic resection of the submandibular gland: a new approach. Head Neck. 2006 Nov;28(11):1014-7.
29. Roh JL. Removal of the submandibular gland by a retroauricular approach. Arch Otolaryngol Head Neck Surg. 2006 Jul;132(7):783-7.
30. Quer M, Vander Poorten V, Takes RP, Silver CE, Boedeker CC, de Bree R, et al. Surgical options in benign parotid tumors: a proposal for classification. Eur Arch Otorhinolaryngol. 2017 Nov;274(11):3825-36.
31. Eveson JW, Cawson RA. Salivary gland tumours: a review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985 May;146(1):51-8.
32. Albergotti WG, Nguyen SA, Zenk J, Gillespie MB. Extracapsular dissection for benign parotid tumors: a meta-analysis. Laryngoscope. 2012 Sep;122(9):1954-60.
33. O’Brien CJ. Current management of benign parotid tumors: the role of limited superficial parotidectomy. Head Neck. 2003 Nov;25(11):946-52.
34. Zhi K, Ren W, Gao L, Zhao L, Huang S, Li J, et al. Face-lift incision combined with sternomastoid muscular flap in parotidectomy. Aesthetic Plast Surg. 2011 Aug;35(4):558-62.
35. Roh JL. Extracapsular dissection of benign parotid tumors using a retroauricular hairline incision approach. Am J Surg. 2009 May;197(5):e53-6.
36. Lin SD, Tsai CC, Lai CS, Lee SS, Chang KP. Endoscope-assisted parotidectomy for benign parotid tumors. Ann Plast Surg. 2000 Sep;45(3):269-73.
37. Li Y, Xue R, Lai Q, Xu B, Yuan K, Tang X, et al. Endoscope-assisted resection of nonneoplastic space-occupying lesion in oral and maxillofacial areas. Sci Rep. 2017 Dec;7(1):16920.
38. Zhong LP, Zhao SF, Chen GF, Ping FY. Ultrasonographic appearance of lipoma in the oral and maxillofacial region. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Dec;98(6):738-40.
39. Miyakubo M, Oriuchi N, Tsushima Y, Higuchi T, Koyama K, Arai K, et al. Diagnosis of maxillofacial tumor with L-3-[18f]-fluoro-alpha-methyltyrosine (FMT) PET: a comparative study with FDG-PET. Ann Nucl Med. 2007 Feb;21(2):129-35.
40. Glosser JW, Pires CA, Feinberg SE. Branchial cleft or cervical lymphoepithelial cysts: etiology and management. J Am Dent Assoc. 2003 Jan;134(1):81-6.
41. Tanaka N, Murata A, Yamaguchi A, Kohama G. Clinical features and management of oral and maxillofacial tumors in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999 Jul;88(1):11-5.
42. Mitroi M, Dumitrescu D, Simionescu C, Popescu C, Mogoanta C, Cioroianu L, et al. Management of second branchial cleft anomalies. Rom J Morphol Embryol. 2008;49(1):69-74.
43. Ahn Y, Lee SH. Laser-assisted transforaminal endoscopic lumbar discectomy: technical pearls for prevention of complications. Med Lasers. 2013 Dec;2(2):43-8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







