 |
 |
- Search
AbstractObjectivesNeck recurrence of papillary thyroid cancer (PTC) is frequently detected after initial surgery. The management of these lesions may include rescue surgery (RS) or minimally invasive techniques in selected patients, but comparative studies evaluating the effectiveness and safety of these techniques are lacking. In this paper, we compared ultrasound-guided ethanol ablation (EA) in selected patients to RS in a matched cohort.
MethodsWe retrospectively compared 41 patients and 41 matched PTC patients without known distant metastases, who underwent ultrasound-guided EA or RS (matched reference group), who had 63 and 75 thyroid bed and/or lymph node confirmed PTC recurrences during a median follow-up of 72.8 and 89.6 months, respectively. The primary outcome was time until structural recurrence, compared using Kaplan-Meier survival curves. The secondary outcomes included time until biochemical recurrence, plasma thyroglobulin (Tg) levels, American Thyroid Association (ATA) response-to-therapy categories by the last available observation, and treatment-derived complications in each group.
ResultsNo significant differences were found between the EA and RS groups for time until structural recurrence (log-rank test, P=0.94). The time until biochemical recurrence was also similar (P=0.51); and the plasma Tg concentration reduction and proportions of patients in the ATA reclassification categories were also similar. A significantly higher proportion of patients in the RS group presented treatment-derived complications (29.27% vs. 9.75%, P<0.05).
ConclusionIn this retrospective analysis, the treatment of PTC neck recurrence with EA in selected patients was comparable to RS in a matched reference group for the long-term risk of structural or biochemical relapse, but with a lower risk of treatment-derived complications. These results support the effectiveness and safety of this minimally invasive technique in the management of selected patients with recurrent PTC.
Over the past few decades, the global incidence of differentiated thyroid cancer (DTC) has been steadily increasing, with estimates as high as nine and three cases per 100,000 person-years in the female and male populations, respectively [1]. Despite the generally excellent prognosis, with disease-specific mortality rates less than 1 per 100,000 person-years [1], a significant number of patients will experience recurrent or persistent structural disease following initial treatment. According to the American Thyroid Association (ATA) risk of recurrence staging system [2], up to 5%, 6 to 20%, and over 20% of patients classified as having low, intermediate, or high risk of recurrence, respectively, will experience persistent disease or relapse, potentially necessitating further intervention.
In most cases, formal rescue surgery (RS) of the affected neck compartments is considered the primary treatment. However, this approach only results in biochemical remission in 21% to 61% of these patients [3,4], and the risk of complications associated with reintervention is high due to the distortion of neck anatomy resulting from scar tissue formation, particularly in patients who have undergone repeated neck dissections [5,6] and/or those involving the central compartment [7].
Additionally, the negative impact of further surgery on quality of life, as well as the patientãs reluctance to undergo repeat surgery after a previous neck dissection, are important considerations given the often slow progression of DTC lymph node recurrence. For these reasons, current thyroid cancer guidelines suggest considering active surveillance for cervical nodal metastases with a diameter of 10 mm or less that appear stable or slow-growing [2]. However, patients considered for this approach must be reliable and consistent with follow-up and must be involved in the decision-making process [3].
As an alternative, several image-guided minimally invasive techniques (MITs), including ethanol ablation (EA) and thermal ablation, have been proposed as potential outpatient therapies for incidentally discovered indolent cervical recurrences of papillary thyroid cancer (PTC). These techniques aim to provide a balanced option between active surveillance and surgical reintervention [8]. Numerous publications have provided multidisciplinary international clinical guidance for the appropriate use of ultrasound (US)-guided MITs in malignant thyroid lesions, including indications for EA use in patients with neck recurrence of PTC [8-10]. However, one of the main weaknesses identified by these consensus documents is the lack of studies directly comparing the long-term outcomes of MITs versus RS.
In this paper, we present a retrospective observational study that assesses the long-term effectiveness and safety of EA in selected patients for the treatment of neck recurrence of PTC, compared to RS performed in a cohort of patients with similar baseline characteristics. The primary outcome of this study was the time until structural recurrence throughout the follow-up period. Secondary outcomes included the time until biochemical recurrence, the proportion of patients falling into ATA 1ã4 responseto-therapy reclassification categories [2], changes in plasma thyroglobulin (Tg) concentration compared to baseline at the last available observation, and complications arising from the treatment. The aim of this research was to provide long-term evidence on the role of EA in selected patients with neck recurrence of PTC as a potential alternative to surgery.
Fig. 1 shows patient disposition in this study. A total of 964 patients with a diagnosis of thyroid cancer (821 with PTC) were registered in the Balearic Islands public hospitals database from January 2013 to December 2020. Up to 52 patients (5.39%) underwent US-guided percutaneous EA, either for curative or palliative intention. Of them, 41 patients (4.25%) with a diagnosis of neck recurrent PTC were included in this study. Patients with other than PTC, known distant metastases or insufficient follow-up data were excluded. Patients with EA procedure performed with a palliative intention were also excluded from this study. EA procedures were performed on a total of 63 lesions identified either as thyroid bed recurrence or lymph node metastases by fine needle aspiration (FNA) cytology and/or measurement of Tg in FNA washouts. Conversely, a retrospective review of the same database identified 95 patients (9.85%) with PTC thyroid bed recurrence and/or lymph node involvement, who underwent RS for the same period of time and 41 patients (4.25%) with 75 confirmed lesions were finally included as a reference group after matching for: baseline characteristics (age, sex, and risk factors for DTC); tumoral burden (tumor size, TNM postoperative stage, ATA risk of recurrence category and baseline plasma Tg levels) and initial management (lateral neck dissection and radioactive iodine [RAI] ablation therapy). Patients with known distant metastases were excluded from this analysis (six patients in which EA was performed for palliative purposes and eight patients who underwent neck surgery either for palliative purposes or aiming to reduce tumoral burden). Patients with previous RS who underwent further EA (seven patients) were also excluded from analysis. The decision to undergo EA versus active surveillance or RS was taken in a multidisciplinary committee, according to the best clinical judgement and based upon accepted international guidelines, including the following inclusion criteria: (1) high-risk, contraindication for, or patient decline of surgery; (2) a number equal or less than three confirmed neck lesions, of less than 2 cm in the greater diameter and (3) that are accessible to US-guided needle puncture [8-10].
The study protocol was reviewed and approved by the Institutional Ethics Committee of the Balearic Islands (CEIC-IB; IB/4812/22). All patients gave written informed consent as part of routine requisites before undergoing EA procedures.
EA procedures were performed by two experienced endocrinologists (ST, IA), according to the widely accepted technique proposed by Shin et al. [10,11]. Preliminary results of our cohort, including 42 patients with an average follow up of 40.5 months, have been previously published [12], and Supplementary Table 1 provides detailed results of EA procedures included in the present study. Essentially, treated lesions were considered successfully ablated when a volume reduction >50% of baseline volume with absence of intranodal power Doppler signal was confirmed by follow-up US and Tg determination in FNA washout was <1 ng/mL, all taken together. Conversely, RS was performed by surgery teams in all five public hospitals across Balearic Islands.
Hospital records were reviewed and baseline characteristics of patients and primary tumors were recorded, including sex, age at diagnosis, risk factors for thyroid cancer, histological type, size, TNM stage and ATA risk of recurrence stratification category, extent of primary surgery, RAI remnant ablation therapy and RAI dose. Additionally, number, size and location of neck recurrences were also recorded for each patient, as well as plasma Tg concentration (both with suppressed and stimulated thyroid stimulating hormone [TSH], when available) and anti-Tg antibodies (Tg-Ab), by the time of recurrent disease diagnosis and by the last available observation. All baseline characteristics were used to select a matched reference group aiming to reduce potential differences between both groups of patients regarding risk of recurrence and aggressiveness of primary tumors. Mean and interquartile range duration of follow-up was recorded for each group, starting after RS or last EA procedure had been performed, respectively.
The primary outcome in this study was time until structural recurrence following EA or RS. Kaplan-Meier survival curves were compared between both groups using Log-rank and Wilcoxon tests. Secondary outcomes in this study included: time until biochemical relapse (defined by sustained increase in plasma Tg levels >1 ng/mL); comparison of the proportion of patients falling into 1 to 4 ATA response-to-treatment re-classification categories and plasma Tg concentration (both with suppressed and stimulated TSHãwhen available) and percentage of reduction from baseline by the last available observation. Finally, the proportions of patients with treatment-derived complications were also compared between both treatment groups.
A step-wise regression analysis was performed to identify baseline characteristics associated to risk of recurrence in the whole cohort, and a comparison of them was performed between both groups to confirm adequate matching. Variables with a normal distribution were compared using Student t-test, Wilcoxon test was used for comparison of non-parametrical variables and chi-square test for categorical ones. For all statistical comparisons, a two-tailed level of P<0.05 was assumed for significance.
All baseline characteristics of patients and primary tumors were similar between the EA and RS groups. These characteristics included initial lateral neck involvement, histology, postoperative TNM stage, ATA risk of recurrence categories, and the proportion of patients receiving RAI therapy and RAI dose (Table 1).
Regarding the baseline characteristics of PTC neck recurrence, the time interval between primary surgery and neck recurrence was significantly longer in patients who underwent EA. A significantly larger proportion of lesions located in the central compartment or thyroid bed were treated with EA, while a significantly larger proportion of latero-cervical lesions underwent RS. Lastly, the recurrent tumoral burden, as indicated by baseline TSH-suppressed and TSH-stimulated plasma Tg concentrations, was similar between both patient groups (Table 2).
Fig. 2A presents the Kaplan-Meier survival curves for the time to structural relapse in both the EA and RS groups. Structural relapse was defined by the detection of new or persistent disease using either anatomical (computed tomography scan or US) or functional (RAI or 18fluorodeoxyglucose [18FDG]-avid lesions pm a whole body scan or positron-emission tomography) imaging. The log-rank and Wilcoxon tests revealed no significant differences in structural relapse between patients who underwent EA and those who underwent RS during the follow-up period. An additional sub-analysis was performed according to the initial postoperative TNM stage and ATA risk of recurrence (Supplementary Figs. 1 and 2). In neither case was a significant difference in time until structural relapse found across all groups. The management of structural and biochemical relapse in both groups is also described in the Supplementary Tables 2 and 3.
Fig. 2B presents the Kaplan-Meier survival curves, illustrating the time to biochemical relapse for both the EA and RS groups. Biochemical relapse was characterized by either the persistence or increase of plasma Tg levels exceeding 1 ng/mL (with suppressed TSH) or surpassing 10 ng/mL (with stimulated TSH), depending on the available data. Patients with positive Tg-Ab were not included in this comparative analysis. Once again, no significant differences were identified in the risk of biochemical relapse between the EA and RS groups.
No significant differences were observed in the percentage of patients who developed new recurrences in the same neck compartment after undergoing either EA or RS. A significantly larger proportion of patients received RAI therapy after RS compared to those in the EA group, even though the RAI dose was significantly higher in the latter group. However, plasma Tg concentrations decreased similarly in both patient groups during follow-up, regardless of whether TSH was suppressed or stimulated. Furthermore, a comparable proportion of patients were reclassified into ATA 1 to 4 response-to-therapy categories (Table 3, Supplementary Fig. 1).
Finally, a higher proportion of complications were observed in the RS group (P=0.03), which included transient or persistent damage to the recurrent laryngeal nerve (RLN) and transient hypocalcemia. Other complications encompassed hematoma that required surgical drainage, surgical infection, two instances of persistent Claude-Bernard-Horner syndrome, and a case of bilateral diaphragmatic palsy associated with respiratory insufficiency. In the EA group, complications were confined to four instances of transient RLN damage, which resulted in hoarseness and/or voice fatigue. However, all these cases recovered within a few days to weeks. A notably thin 38-year-old woman reported experiencing chest pain and discomfort for 2 months following the ablation of a pre-tracheal lesion, likely caused by alcohol leakage (Table 4). During EA procedures, varying degrees of pain were experienced by up to 30% of patients, although none were severe or persistent.
In this paper, we present a multicentric retrospective study comparing EA, an MIT intended to avoid reintervention in selected patients with recurrent PTC in the neck, versus RS, the classically recommended first-line treatment for these patients. After matching for baseline patient demographics and tumor characteristics, including plasma Tg levels, we found no difference in the time to new structural or biochemical relapse between patients who underwent EA and those who underwent RS during the follow-up period. Furthermore, we detected no differences in long-term plasma Tg reduction compared to baseline levels, despite a significantly lower proportion of patients receiving RAI therapy after EA compared to RS. Lastly, the proportion of patients categorized into the ATA lower risk response-to-treatment dynamic reclassification categories of ãexcellentã and ãindeterminate response,ã and the higher risk categories of ãstructuralã and ãbiochemical incomplete response,ã were similar between both groups based on the last observation available for each patient. This confirms that the long-term outcomes of EA in selected patients are comparable to those of RS.
Treatment-derived complications occurred more frequently in the RS group, with some persisting and thereby increasing the morbidity of reintervention surgery. Of note, a larger number of patients with central compartment or thyroid bed recurrences underwent EA rather than RS. In our experience, central compartment involvement is a significant factor that favors MITs over RS, in order to prevent injury to the RLN and parathyroid glands, as the risk for these complications is higher in a neck that has previously been operated on. In fact, even though the proportion of central compartment or thyroid bed recurrences was higher in the EA group, RLN injury was documented in only three patients. All of these patients had recurrent disease involving the central compartment, but in all cases, symptoms such as hoarseness and voice fatigue fully resolved. All serial EA procedures were safely performed in an outpatient setting.
To the best of our knowledge, this is the first multicentric comparative study that evaluates the long-term outcomes of EA in selected patients versus RS in cases of recurrent thyroid cancer in the neck. Several authors have previously documented their experiences with EA for treating recurrent thyroid cancer in the neck, in series including a variable number of patients, treated lesions, and follow-up durations [11-18]. Overall, successful ablation rates range from 85% to 98%, with re-recurrence rates as low as 2.4% of treated lesions [17]. EA has demonstrated its effectiveness and safety, with its impact on disease progression measured by a reduction in plasma Tg concentrations ranging from 71% [18] to 93.8% [17]. In all these studies, EA was offered to selected patients with a limited number of identifiable lesions, and/or those at high risk or unwilling to undergo reoperation. A retrospective study and a meta-analysis have compared the effectiveness and safety of EA versus RFA [16,17]. Both studies concluded that both techniques are equally effective and safe for treating neck recurrences, with a non-significant higher proportion of re-recurrence and a lower rate of treatmentderived complications in lesions treated with EA. Notably, neither of these studies evaluated long-term survival free of structural or biochemical relapse. In 2015, Fontenot et al. [18] published a systematic review and pooled analysis comparing EA and RS for treating locally recurrent PTC. The paper included a total of 27 published studies, with 168 (11.4%) lesions treated with EA and 1,449 (88.6%) treated with RS (reintervention). The study found a significantly higher rate of success in treating lesions with RS versus EA (94.8% vs. 87.5% of cases; odds ratio [OR], 2.58; 95% confidence interval [CI], 1.55ã4.31; P<0.001). However, the recurrence rates for EA and RS at the site of the treated lesion or elsewhere in the neck were similar (11.9% vs. 12.7%; OR, 1.07; 95% CI, 0.65ã1.77; P=0.78). Reoperation was associated with a 3.5% pooled risk of complications, while EA incurred a pooled risk of 1.2% (OR, 2.9; 95% CI, 0.72ã 12.3; P=0.08). Interestingly, patients in the EA group had undergone more previous operations (2.1) compared to those in the RS group (1.03), which could reflect a potential selection bias increasing recurrence risk in the EA group. Similar to our study, patients in the EA group were treated for a median of 1.6 lesions, with a median lesion volume of 0.45 mL. Furthermore, consistent with our study, plasma Tg concentrations were reduced on average by 73% in the RS group and 71% in the EA group, although the mean follow-up period was shorter than in our study: 31.1 months in studies evaluating reintervention and 32.9 months in those evaluating EA. This paper did not address survival curves for structural and/or biochemical relapse. However, the large overlap in both baseline characteristics of patients and outcomes after EA and RS, respectively, likely reflects a high level of agreement in patient selection for EA procedures, further validating our results.
Our study has a significant limitation due to its observational nature. Patients undergoing EA must meet several criteria, including a high risk or refusal for surgery on one hand, and a limited number and size of detected lesions on the other. Therefore, we cannot entirely rule out a selection bias between the two patient groups, even after baseline matching. Consequently, our results should be interpreted with caution. The purpose of this paper was to evaluate the effectiveness of EA compared to RS, but only within this specific subset of selected patients. The results cannot be generalized to all patients with recurrent PTC in the neck. Additionally, the higher prevalence of central compartment lesions in the EA group likely reflects a preference to avoid reintervention in this neck compartment to prevent potential injury to the RLN and/or the parathyroid glands. This is particularly true for patients who have previously experienced complications. Lastly, we cannot rule out a bias related to the higher proportion of patients receiving RAI therapy after RS, which could indicate a more aggressive disease course in these patients. However, the long-term decrease in plasma Tg concentrations was largely similar in both groups. A sub-analysis according to both initial TNM and ATA risk of recurrence did not show statistically significant differences. Furthermore, the management of structural or biochemical re-recurrence was not different between the two groups.
Nonetheless, our study has several strengths. First, the effectiveness and safety of EA in our series were comparable to those previously published, making it suitable for long-term comparisons with RS. Second, unlike the meta-analysis by Fontenot et al. [18], we present long-term data regarding re-recurrence rates, both structural and biochemical, suggesting that both procedures have a similar impact on the disease course. Finally, based on the specific recommendations of guidelines regarding the indications of EA in recurrent thyroid cancer in the neck, large prospective randomized trials may not be feasible. However, adherence to a rational treatment protocol within the context of a multidisciplinary committee, as was the case in our study, may allow for valuable comparisons to standard care. In this regard, our study results largely align with the effectiveness rates reported by previous authors for EA and the long-term outcomes reported in the paper by Fontenot et al. [18].
In conclusion, this study is the first to retrospectively compare EA in selected patients with RS in a matched reference group for the management of recurrent PTC in the neck. The results confirm the effectiveness of this MIT as an alternative to reoperation, with a lower risk of treatment-related complications and a long-term risk of re-recurrence. We believe that in the era of individualized treatment strategies for managing recurrent thyroid cancer, effective and safe MITs like EA will become significant treatment options. These should be considered in the new patient-centered decision-making process.
㈠Neck recurrence of papillary thyroid cancer is frequent, although the disease-specific mortality of this cancer is low. In this context, a balanced approach to patients with recurrent disease is essential to minimize treatment-related complications.
㈠The management of recurrent disease with minimally invasive techniques (MITs) can be safe and effective, and MITs can potentially reduce complications arising from rescue surgery, although comparative studies are lacking.
㈠In this retrospective observational comparison, ethanol ablation performed in selected patients offered similar results for disease-free survival, with lower complications than rescue surgery in a matched cohort.
NotesAUTHOR CONTRIBUTIONS Conceptualization: ST, IA, VP. Data curation: ST, IA, CA, AR. Formal analysis: ST, AT, AB, VP. Investigation: ST, IA, AT, VP. Methodology: ST, AT, AB, VP. Project administration: ST. Resources: ST. Supervision: ST, AT, AB, VP. Validation: ST, AT, AB, VP. Visualization: ST. Writingãoriginal draft: ST. Writingãreview & editing: ST, AB, VP. ACKNOWLEDGMENTSThe authors wish to deeply thank the colleagues from the Endocrinology Departments of the Public Health System Hospitals in the Balearic Islands for their valuable contribution to the data search and to our patients, to whom this paper is dedicated.
Supplementary materialsSupplementary materials can be found online at https://doi.org/10.21053/ceo.2023.00689.
Supplementaryô Tableô 2.Analysis of neck structural re-recurrence (patients) after percutaneous ethanol injection vs. rescue surgery Supplementaryô Tableô 3.Management of structural and/or biochemical re-recurrence or persistence (patients) after ethanol injection vs. rescue surgery Supplementaryô Fig.ô 1.(A-D) Kaplan-Meier survival curves for time to structural relapse (patients) after ethanol injection vs. rescue surgery, according to initial TNM stage. S(t) means survival as a function of time. Supplementaryô Fig.ô 2.(A-C) Kaplan-Meier survival curves for time to structural relapse (patients) after ethanol injection vs. rescue surgery, according to initial American Thyroid Association (ATA) risk of recurrence stage. S(t) means survival as a function of time. Fig.ô 1.Disposition of patients with papillary thyroid cancer (PTC). Propensity score matching included patient demographics, followup duration, tumoral burden measured by number and size of lesions, primary tumor histology, and plasma thyroglobulin levels. DTC, differentiated thyroid cancer; PEI, percutaneous ethanol injection; RS, rescue surgery; EA, ethanol ablation. 
Fig.ô 2.Kaplan-Meier survival curves for (A) time to structural relapse and (B) time to biochemical relapse in patients undergoing ethanol ablation versus rescue surgery. Statistical comparison of survival curves. S(t) means survival as a function of time. Log-rank test: (A) P=0.94, (B) P=0.52; Wilcoxon test: (A) P=0.86, (B) P=0.48. 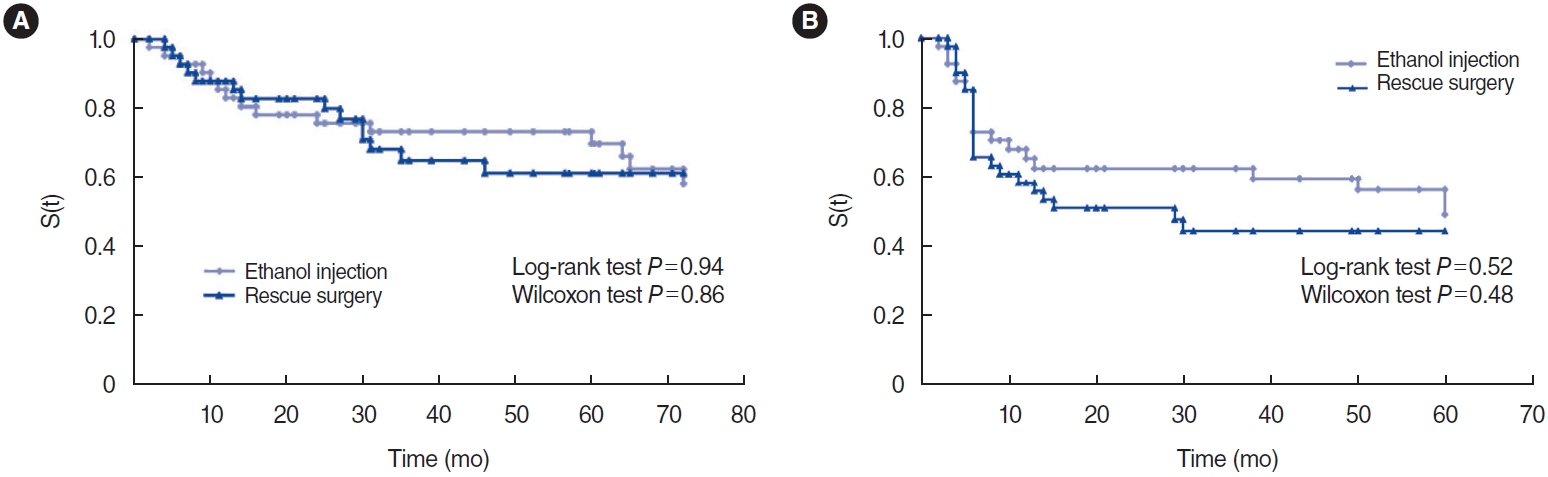
Tableô 1.Baseline characteristics of matched patients and their primary tumors
Tableô 2.Baseline characteristics of papillary thyroid cancer neck recurrences Tableô 3.Results of ethanol ablation versus rescue surgery by last available observation Tableô 4.Treatment-derived complications following ethanol ablation or rescue surgery
REFERENCES1. European Network of Cancer Registries (ENCR). ENCR factsheets [Internet]. ENCR; 2017 [cited 2023 Sep 1]. Available from: https://www.encr.eu/sites/default/files/factsheets/ENCR_Factsheet_Thyroid_2017-2.pdf.
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26(1):1-133.
3. Urken ML, Milas M, Randolph GW, Tufano R, Bergman D, Bernet V, et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the Thyroid Cancer Care Collaborative. Head Neck. 2015 Apr;37(4):605-14.
4. Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab. 2010 May;95(5):2187-94.
5. Uchida H, Imai T, Kikumori T, Hayashi H, Sato S, Noda S, et al. Longterm results of surgery for papillary thyroid carcinoma with local recurrence. Surg Today. 2013 Aug;43(8):848-53.
6. Roh JL, Kim JM, Park CI. Central compartment reoperation for recurrent/persistent differentiated thyroid cancer: patterns of recurrence, morbidity, and prediction of postoperative hypocalcemia. Ann Surg Oncol. 2011 May;18(5):1312-8.
7. Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992 Sep;75(3):714-20.
8. Mauri G, Hegedus L, Bandula S, Cazzato RL, Czarniecka A, Dudeck O, et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021 Jun;10(3):185-97.
9. Hahn SY, Shin JH, Na DG, Ha EJ, Ahn HS, Lim HK, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2019 Apr;20(4):609-20.
10. Orloff LA, Noel JE, Stack BC, Russell MD, Angelos P, Baek JH, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: an international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. 2022 Mar;44(3):633-60.
11. Shin JE, Baek JH, Lee JH. Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: current status and challenges. Curr Opin Oncol. 2013 Jan;25(1):14-9.
12. Tofe S, Arguelles I, Serra G, Garcia H, Barcelo A, Pereg V. Ultrasound-guided percutaneous ethanol ablation for the management of recurrent thyroid cancer: evaluation of efficacy and impact on disease course. Int J Thyroidol. 2020 Nov;13(2):128-41.
13. Heilo A, Sigstad E, Fagerlid KH, Haskjold OI, Groholt KK, Berner A, et al. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011 Sep;96(9):2750-5.
14. Lewis BD, Hay ID, Charboneau JW, McIver B, Reading CC, Goellner JR. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol. 2002 Mar;178(3):699-704.
15. Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW. Longterm outcome of ultrasound-guided percutaneous ethanol ablation of selected ãrecurrentã neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013 Dec;154(6):1448-55.
16. Guenette JP, Monchik JM, Dupuy DE. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol. 2013 May;24(5):672-9.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







