 |
 |
- Search
AbstractObjectives. Laryngeal ultrasonography (LUS) has been suggested as an alternative diagnostic tool for unilateral vocal fold paralysis (UVFP). The present study applied LUS and quantitative laryngeal electromyography (LEMG) in female UVFP patients to investigate the pathophysiologic mechanisms of UVFP.
Methods. In this cross-sectional study, vocal fold (VF) length parameters included resting and phonating VF length measured using B-mode LUS, and color Doppler vibrating length (CDVL) measured using the color Doppler mode.
Results. Forty female patients with UVFP were enrolled, among whom 11 and 29 were assigned to the thyroarytenoid (TA) muscle+cricothyroid (CT) muscle group (with CT involvement) and the TA (without CT involvement) group, respectively. In the TA group, the turn frequency in thyroarytenoid-lateral cricoarytenoid (TA-LCA) on the paralyzed side, as observed through LEMG, correlated with the VF length during the resting phase (R=0.368, P=0.050) and CDVL values (R=0.627, P=0.000) on the paralyzed side. In the TA+CT group, the turn ratio in the CT muscle correlated with the normalized phonatory vocal length change (nPLC; R=0.621, P=0.041) on the paralyzed side.
Conclusion. CDVL and nPLC are two parameters that can be utilized to predict the turn frequencies of TA-LCA in UVFP cases without CT involvement, and the turn ratio of CT in cases of UVFP with CT involvement, respectively. The findings suggest that LUS, as a noninvasive tool, can serve as an alternative method for assessing the severity of laryngeal nerve injury and offer valuable insights into the pathophysiology of UVFP.
Unilateral vocal fold paralysis (UVFP) refers to limited vocal fold (VF) movement caused by neurogenic pathology, and its common etiologies include iatrogenic injuries, malignancies, and idiopathic paralysis. Thyroid surgery is the most common procedure that causes UVFP [1,2]. UVFP affects the ability to communicate during life and at work, further causing social consequences and depression [3]. Therefore, identifying and evaluating disease severity early for a prompt intervention are crucial.
Concurrent neuropathy of the superior laryngeal nerve (SLN) may also be present, manifesting as paralysis of the cricothyroid (CT) muscle. Previous studies have shown that additional SLN involvement in UVFP may induce poorer speech performance and quality of life, resulting in a worse trajectory of outcomes [4]. In our previous study, patients with CT involvement had poorer VF vibration and decreased aerodynamic parameters [5,6]. Compared with UVFP without CT involvement, voice quality and quality of life were more severely impaired in patients with CT paralysis [5]. These findings support that the CT muscle plays an important role in physiological vocalization, and its functional implications in UVFP must be emphasized. A spectrum of evaluation tools has been applied for evaluating the functional effects of SLN paralysis, such as symptomatic evaluation, stroboscopy, acoustic examination, and laryngeal electromyography (LEMG). LEMG is the objective gold-standard tool for evaluating the level of recruitment limitation in the CT muscle caused by SLN paralysis. However, electromyography is not routinely performed in most clinics, even though evidence has supported its importance, making the diagnosis of SLN paralysis difficult [7].
Laryngeal ultrasonography (LUS) has been proposed as a promising tool for examining VF function [8-10]. Moreover, LUS minimally interferes with phonation, allowing the observation and measurement of VFs to be carried out more naturally. Several studies have used B-mode LUS to measure both the resting and phonating VF length [11,12]. During phonation, the vibration frequency of the VF is regulated by its elasticity, which is determined by its length and tension; therefore, quantifying the VF length parameters is critical for understanding the pathophysiology of UVFP-induced dysphonia. Utilizing Doppler analysis that measures tissue motion along the probe direction [13], Hsiao et al. [13] used colored ultrasonography artifacts to estimate the vibratory motion of the mucosa-air column interface of the VF. However, the associations between the ultrasonographic parameters of the VF and the severity of neuropathy, as well as the impact of SLN involvement on ultrasonographic findings, remain unclear.
The aim of the study was to evaluate the role of LUS, a noninvasive assessment, in UVFP. We hypothesized that severity of denervation of the intrinsic laryngeal muscles may affect the conformation of the VFs, resulting in a shorter resting VF length or reducing phonatory dynamic length change on the paralyzed side.
This cross-sectional study was approved by the Institutional Review Board of the Chang Gung Memorial Foundation (No. 201401840B0). Written informed consent was obtained from each patient. Consent was also obtained from the colleague in the demonstration photo. A total of 65 patients diagnosed with UVFP that without surgical treatment were enrolled at a single medical center from September 2014 to December 2017. Patient were included if LEMG was performed within 6 months from UVFP onset. Twenty-five patients were excluded, among whom three had a time from UVFP onset to LEMG examination longer than 6 months, and four had incomplete data. All the male patients (n=18) were excluded because of the difficulties in measuring VFs using LUS, which may be attributed to ossification of thyroid cartilage in male patients. Finally, forty female patients with UVFP were enrolled. Eleven patients with concomitant denervation in the ipsilateral CT muscle confirmed by LEMG were assigned to the thyroarytenoid (TA) muscle+CT muscle group. Twenty-nine patients without CT muscle denervation were assigned to the TA group (Fig. 1). Each patient received comprehensive assessments, including videolaryngostroboscopy, LEMG examination with quantitative analysis, and LUS of the VFs under the B-mode and color Doppler mode.
Needle LEMG examination using a Nicolet Viking Select system (Cardinal Health) was performed by a board-certified otolaryngologist (TJF) and physiatrist (YCP). Using a concentric needle electrode and a surface ground electrode adhered to the forehead, LEMG signals for bilateral thyroarytenoid-lateral cricoarytenoid (TA-LCA) muscle complexes and CT muscles were obtained, with the bandpass filter set between 20 Hz and 10 kHz. Next, 2 mL of 2% lidocaine hydrochloride was injected into the subcutaneous tissue at the LEMG needle-insertion sites to avoid discomfort. For the TA-LCA muscle complexes, patients were asked to produce three series of /e/ sounds at three different intensities (low, moderate, and highest possible). For the CT muscles, the patients produced three series of glissando upward /e/ sounds at normal loudness. In the context of qualitative evaluation, the presence of spontaneous activities (such as fibrillation, positive sharp wave, and complex repetitive discharge), polyphagia exceeding 30%, or a decreased recruitment pattern (characterized by reduced, discrete, or no interference pattern) detected through LEMG suggests the involvement of the TA-LCA complex or CT muscle (Supplementary Video 1). For quantitative analysis, a MATrix LABoratory-based program (The MathWorks) was developed to analyze the raw LEMG data. Raw LEMG waveforms were first binned into nonoverlapping epochs, and the epoch durations for the TA-LCA muscle complexes and CT muscle were 200 [5] and 50 ms [14], respectively.
The timing and amplitude of each turn were calculated using an automatic algorithm. The epoch durations for the TA-LCA and CT muscles were 200 and 50 ms, respectively (Supplementary Fig. 1A). A turn was defined by the change in polarity with an amplitude of at least 100 µV before and after the change to exclude noise-related peaks. The turn frequency was computed for each epoch as the number of turns divided by the epoch duration, and the peak turn frequency was the mean of the highest three turn frequencies for all the epochs obtained in each muscle (Supplementary Fig. 1B). To prevent individual differences, the turn ratio was defined as the turn frequency of the paralysis-side muscle divided by the turn frequency of the healthy-side muscle.
Ultrasonographic examination was performed using an Acuson P300 machine (Siemens) with a 6–11 MHz linear array transducer. The patients were seated on a reclining laryngeal examination chair with their neck extended and head supported by an adjustable neck-head set (Fig. 2A). The thyroid cartilage lamina was used as the acoustic window. The transducer was placed parallel to the VF over the thyroid cartilage lamina “one side at a time” (Fig. 2B). On the ultrasonographic image, the VF length is defined as the distance between the anterior commissure and arytenoid cartilage under the B mode. Each parameter was measured in 15 consecutive frames during phonation in each patient and then the data were averaged. The hyperechoic dot underneath the thyroid cartilage represents the anterior commissure, the point where bilateral VFs meet. The arytenoid cartilage, where the posterior end of the VF is attached, is shown as hyperechoic structures behind the VF (Fig. 2C). The length of the VF was measured under the B mode during the resting and phonation phases, yielding the resting VF length and phonating VF length, respectively. During phonation, patients were asked to produce the vowel /a/ at a comfortable pitch and loudness. The dynamic change of the VF is defined by the difference of the VF length between the resting and phonatory phases. In order to standardize the measurement, the normalized phonatory vocal length change (nPLC) was computed using the following equation:
The length of the vibrating part of the VF can be measured by color Doppler imaging [13]. In the color mode setting, the pulse-repetition rate was 11,100 Hz, and the velocity range was set between 0 and 1.22 m/sec. The attachment of VFs to the anterior commissure of the larynx was identified as a gray point, and then a white line on the interface between the true VF mucosa and air column could be identified.
The patient was then asked to produce the vowel /a/ at a comfortable pitch and loudness. During phonation, the color strip artifacts could be detected in the color mode and were generated by the vibration of the air-mucosa interface. The anterior end of the vibrating VF was the point of attachment to the anterior commissure. Its posterior end was the crossing point between the air-mucosa interface and lateral boundary of the color artifact contour. The distance between the anterior and posterior points of the vibrating part of the VF was determined as the color Doppler vibrating length (CDVL) (Fig. 2D).
IBM SPSS for Mac 25.0 (IBM Corp.) was used for statistical analysis. The data were expressed as means±standard deviation. Student t-test was used to compare parametric data, and chi-square test was used to compare categorical data between the TA and TA+CT groups. The Pearson correlation test was used to analyze relationships between variable pairs. Significance was set as a P<0.05. Weak, moderate, and strong correlations were defined by the absolute values of r of 0.2–0.39, 0.40–0.59, and 0.6–0.79, respectively [15].
The patient characteristics, time post-paralysis, and etiology of neuropathy are presented in Table 1. The age of the patients was 52.1±13.8 years, with more UVFP occurring on the left side (right:left, 15:25). The TA+CT and TA groups had a comparable age and side of paralysis. The data for LEMG and LUS were collected on the same day. All patients underwent these examinations within 6 months from the onset of symptoms. The mean time interval from symptom occurrence to the examination was 2.9±1.4 months. Thyroidectomy (45%) was the leading cause of UVFP in the present cohort.
During the resting phase, the VF length on the healthy side was significantly longer than that on the paralyzed side (21.70±2.51 vs. 20.87±2.80 mm, P=0.034), but no significant difference was found in the VF length during phonation (19.35±2.26 vs. 20.04±2.83 mm, P=0.058) (Fig. 3). Comparing the dynamic VF change, the healthy side had a significantly greater nPLC than the paralyzed side (0.11±0.06 vs. 0.04±0.03, P<0.001), indicating that the change in the VF length was greater in healthy VFs. However, CDVL, the length of the vibration part of VF, did not differ between the healthy and paralyzed sides (P=0.716).
To investigate our hypothesis that VF conformation may be affected by nerve injury and reflect the severity of neuropathy, we analyzed the correlations between various measurements obtained from LUS and QLEMG. The resting VF length and CDVL on the paralyzed side showed a weak to moderate correlation with the TA-LCA turn ratio (R=0.318, P=0.045; R=0.461, P=0.003), indicating that the severity of recurrent laryngeal nerve (RLN) injury had mild influence in the LUS VF length parameters (Supplementary Tables 1 and 2).
To evaluate the relationship between SLN injury and VF length parameters, correlation tests were performed in the TA and TA+CT groups. The CDVL of the paralyzed-side VF showed a moderate to strong correlation with the TA-LCA turn frequency and TA-LCA turn ratio in the TA group (R=0.627, P=0.000; R=0.576, P=0.001) (Table 2). The nPLC of paralyzed-side VFs showed a strong correlation with the CT turn ratio in the TA+CT group (R=0.621, P=0.041) (Table 3). The findings suggest that dynamic VF length changes between resting and voicing phase can be influenced by the severity of SLN impairment in UVFP patients with concomitant SLN injury.
VF motion is practically evaluated using laryngoscopy during voicing and breathing activities. However, flexible fiberoptic laryngoscopy is an aerosol-generating medical procedure, placing otolaryngologists at high risk for virus spreading during the global pandemic [16]. Concerning the coronavirus disease 2019 (COVID-19) outbreak, institutions worldwide have started to apply LUS to assess UVFP because of the noninvasive, minimal aerosol generation setting [17,18]. Furthermore, LUS provides more accurate information on VF length and vibration status during phonation than other laryngeal examination methods that typically provide only superior views of the larynx [19].
QLEMG of the larynx is a useful tool in measuring the severity of nerve injuries in UVFP. It can also be applied to predict the prognosis of the paralyzed cord. However, it is still not popular because of the technical difficulty and its invasive nature. LUS also has an advantage over LEMG in the context of UVFP, which occurs most commonly after neck surgery, such as thyroidectomy, as it avoids the challenges associated with accessing disrupted neck landmarks following surgery. Based on sonography techniques developed for UVFP patients [8-10], the present study is the first to comprehensively assess the association between the sonographic parameters of VF and severity of neuropathy measured by quantitative LEMG. The findings provide additional support for the theoretical model involving the TA-LCA, CT, and posterior cricoarytenoid (PCA) muscles in the management of paralyzed VFs during inspiration. Furthermore, they highlight the potential of noninvasive LUS examinations as a means to assess the extent of neuropathy in the RLN.
During inspiration, the TA-LCA muscles are inactive, and the PCA muscle contracts to laterally rotate the arytenoid cartilage, elongating the VF and widening the glottis to maintain an open airway. The CT muscle, which is responsible for lengthening the VFs, may also cooperate with the PCA muscle during inspiration to further increase the abduction of the VFs. However, the effect of CT contraction on VF adduction or abduction has not been consistently characterized [20,21]. A completely injured RLN cannot activate the PCA during rest breathing; thus, the VF remains in its cadaveric position, which is shorter than a normal one [22]. The present study found a modest correlation between the TA-LCA turn ratio and the resting length of the VFs on the paralyzed side. This finding lends some support to the hypothesis that PCA contractions are controlled by residual RLN activity. However, there may be a rationale to assume that the resting VF length in a normal VF reflects a balance between adductor, abductor, and CT activation, as the VF is under tension resulting from a balance of all the intrinsic laryngeal muscles. Further investigation of the LEMG findings of PCA is warranted to establish a clearer link between resting VF length and PCA contributions in UVFP.
B-mode LUS has been reported to be a feasible tool for assessing VF movement [23]. However, during phonation, VFs could not be consistently visualized in B-mode due to the rapid changes in the vertical dimension [24]. Color Doppler was superior to B-mode ultrasonography in UVFP evaluation by the phonatory color pattern asymmetry, as first proposed by Ooi et al. [25]. Hsiao et al. [26] furthermore suggested that the color phantom signals of vocal fold color Doppler imaging reflected its vibratory characteristics, and thus, could be used to estimate VF elasticity [27]. The present study found a strong correlation between CDVL and TA-LCA turn ratio on the paralyzed side in sole RLN injury patients. A comparison with the conventional B-mode LUS vocal length measurements in our report showed that CDVL could predict TA-LCA turn frequencies in sole RLN injuries (i.e., the severity of RLN impairment). When assessing the remaining function of RLN in UVFP without CT involvement, we suggest considering the use of CDVL as a viable clinical approach if QLEMG is not accessible at the institution. The CT muscle plays a functional role in pitch adjustment, but the exact impact on UVFP remains unclear, a challenge that is attributed to the difficulty of eliminating the influence of the severity of TA-LCA damage. Previous studies have reported that the CT muscle does not influence the VF position in UVFP patients in the acute or chronic stage [22,28]. In a clinical study, Woodson reported that unilateral CT muscle contraction equally increased the length of both VFs and did not alter the movement of vocal processes; therefore, it is difficult to diagnose CT paralysis solely based on clinical changes in glottal asymmetry observed during current laryngoscopy procedures [29]. Using Bmode LUS in patients with UVFP with concurrent SLN injury, the present study showed a positive correlation between the nPLC of the affected VF and the turn ratio of the CT muscle (i.e., the severity of SLN injury). In patients with CT impairment, a decreased anterior tilting of the laryngeal box further reduced the ability to elongate VFs during phonation. The nPLC measured by B-mode LUS is a robust predictor of residual SLN function measured by QLEMG. To the best of our knowledge, this study is the first to utilize a noninvasive tool to infer severity of SLN involvement in patients with UVFP. For clinical application, when CT muscle involvement is identified, the severity of CT (SLN) injury can be represented by nPLC. Thus, LUS can be utilized in institutes where quantitative LEMG is unavailable.
This study has several limitations. First, the present study only focused on a relatively small sample size of female patients with acute-phase UVFP; therefore, the results may not be representative of the UVFP population. Second, the baseline demographic data between the TA and TA+CT groups also exhibited a significant difference in the time interval from lesion occurence to the LEMG examination, possibly influencing the level of denervation changes due to the reinnervation effect. Third, the role of synkinesis was not considered. Synkinetic reinnervation was thought to maintain VF position and influence the laryngeal configuration [30]. The impaction of synkinesis was not excluded because there were only two cases in the present study. Fourth, the LUS of the VFs was conducted by a single examiner. We were unable to assess the interobserver reliability in this study. Finally, although LUS provides real-time, noninvasive imaging and accurately yields VF parameters that reflect the severity of neuropathy, quantitative cutoff values for determining the severity of neuropathy have not yet been established.
Color Doppler and B-mode LUS can provide insights into the severity injury in the RLN and SLN. CDVL is a robust predictor of the turn frequencies of TA-LCA in UVFP without CT involvement, while nPLC predicts the turn frequencies of CT in UVFP with CT involvement. LUS is a valuable tool for assessing the severity of neuropathy when QLEMG is not available.
▪ Color Doppler and B-mode laryngeal ultrasonography (LUS) can provide insights into the severity of injuries to the recurrent laryngeal nerve and superior laryngeal nerve.
▪ Color Doppler vibrating length is a robust predictor of turn frequencies of the thyroarytenoid-lateral cricoarytenoid in unilateral vocal fold paralysis (UVFP) without cricothyroid (CT) involvement, while the normalized phonatory vocal length change predicts the turn frequencies of CT in UVFP with CT involvement.
▪ The findings suggest that LUS, as a noninvasive tool, can serve as an alternative method for assessing the severity of laryngeal nerve injuries and offer valuable insights into the pathophysiology of UVFP.
NotesAUTHOR CONTRIBUTIONS Conceptualization: WNL, TJF. Data curation: YAL, YCT, TJF. Formal analysis: YAL, YCT, TJF. Funding acquisition: TJF, YCP. Methodology: WNL, TJF. Project administration: TJF. Visualization: YAL, TJF, YCP. Writing–original draft: YAL, YCT. Writing–review & editing: YAL, YCP, TJF. ACKNOWLEDGMENTSThis research was supported by the Chang Gung Memorial Foundation (No. CMRPG3D1411-3 and CMRPG3K2152 for manpower and analysis, and CMRPG3M0591 for language editing).
The authors also thank Miss Li-Yun Lin and Chain-Fen Chang for collecting the data.
SUPPLEMENTARY MATERIALSSupplementary materials can be found via https://doi.org/10.21053/ceo.2023.01046.
Supplementary Table 1.The raw data of LEMG and laryngeal ultrasonography parameters in 40 female UVFP patients Supplementary Table 2.Correlations between VF length of the paralyzed side and healthy side and QLEMG in all patients Supplementary Fig. 1.(A) A schematic diagram showing the epoch durations. (B) The turn frequency was computed for each epoch as the number of turns divided by the epoch duration, and the peak turn frequency was the mean of the highest three turn frequencies for all the epochs obtained in each muscle. Fig. 1.Experimental flowchart of subject enrollment, exclusion, allocation, and analysis. UVFP, unilateral vocal fold paralysis; LEMG, laryngeal electromyography; TA, thyroarytenoid; CT, cricothyroid. 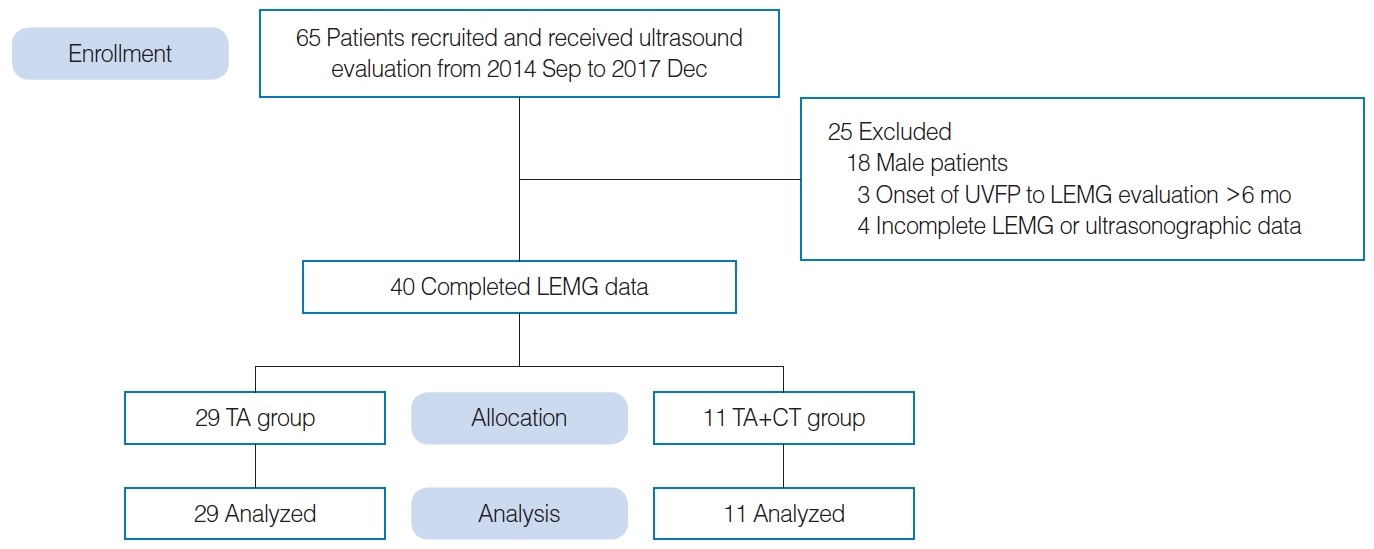
Fig. 2.(A) Patients were seated on a reclining laryngeal examination chair with their neck extended and head supported by an adjustable neck-head set. (B) The transducer was placed transversely over the thyroid cartilage lamina as the acoustic window. (C) The hyperechoic dot located behind the thyroid cartilage represents the anterior commissure (arrow). The arytenoid cartilages are observed as hyperechoic structures behind the vocal folds (VFs; arrowhead). The distance between the anterior commissure and arytenoid cartilages is defined as the VF length. (D) A schematic demonstration of color Doppler vibrating length (CDVL) measurements. During phonation, the anterior end is the point of attachment to the anterior commissure (arrow). The posterior end of the VF is the crossing point between the air-mucosa interface (solid line) and the lateral boundary of the color artefact contour (dashed line). CDVL is as the distance between the anterior and posterior ends. 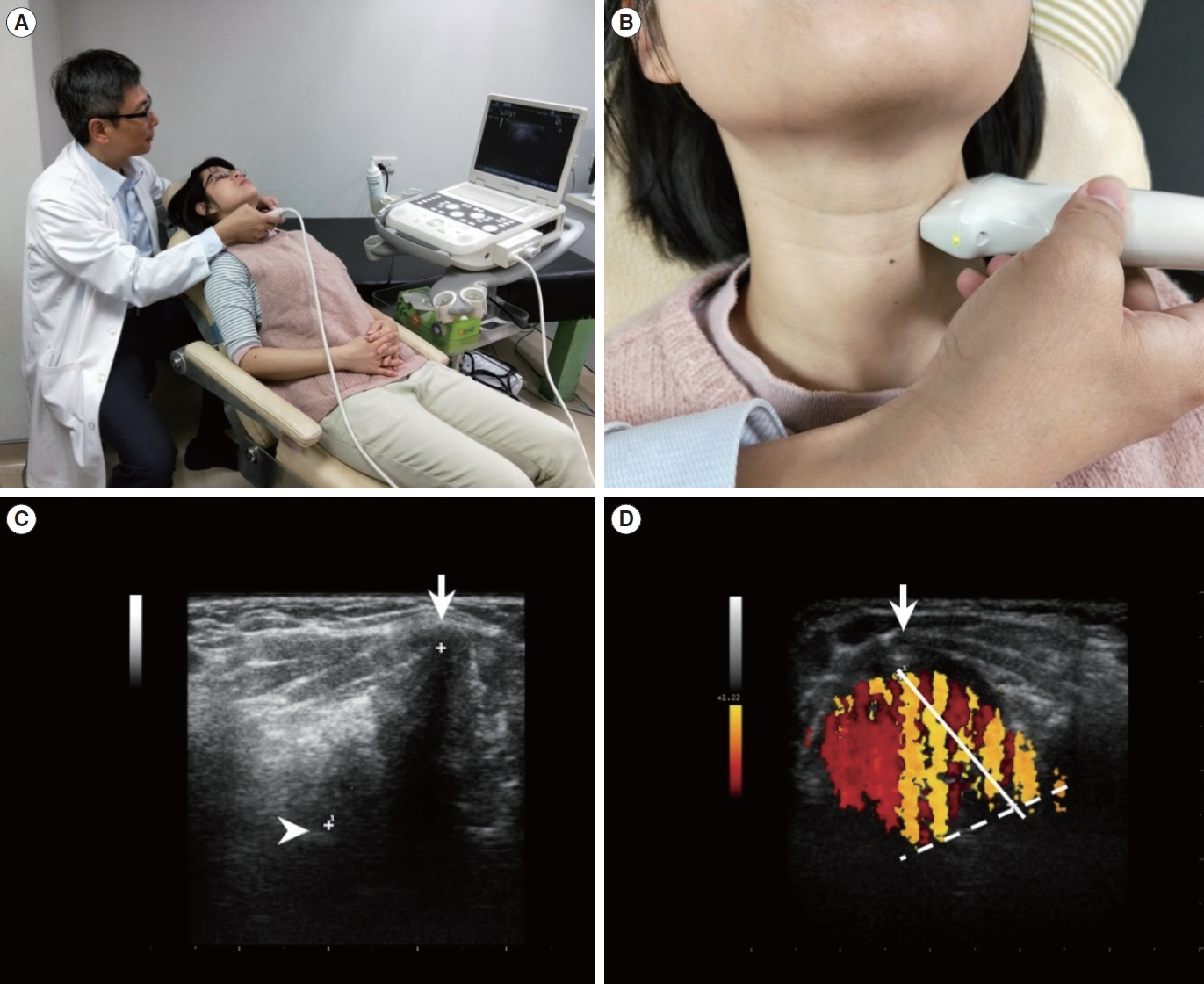
Fig. 3.(A) Vocal fold length on the healthy and paralyzed sides during resting and phonation phase. (B) Normalized phonatory length change (nPLC) on the healthy and paralyzed sides. *P<0.05, ***P<0.01. 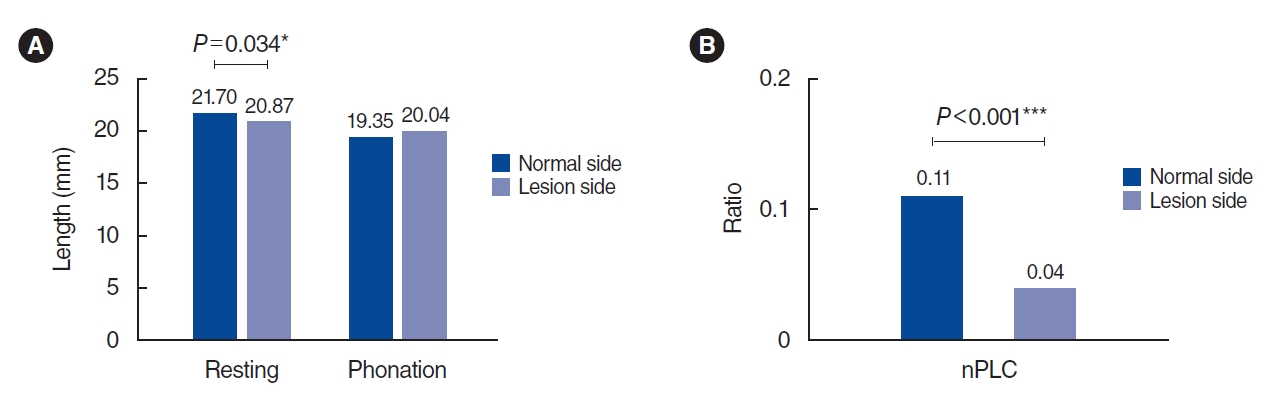
Table 1.Demographics of the UVFP patients
Table 2.Correlation between VF length of the paralyzed side and QLEMG in the TA group (n=29)
Table 3.Correlation between VF length of the paralyzed side and QLEMG in the TA+CT group (n=11)
REFERENCES1. Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope. 2007 Oct;117(10):1864-70.
2. Wang HW, Lu CC, Chao PZ, Lee FP. Causes of vocal fold paralysis. Ear Nose Throat J. 2022 Aug;101(7):NP294-8.
3. Francis DO, Sherman AE, Hovis KL, Bonnet K, Schlundt D, Garrett CG, et al. Life experience of patients with unilateral vocal fold paralysis. JAMA Otolaryngol Head Neck Surg. 2018 May;144(5):433-9.
4. Pei YC, Lu YA, Wong AM, Chuang HF, Li HY, Fang TJ. Two trajectories of functional recovery in thyroid surgery related unilateral vocal cord paralysis. Surgery. 2020 Oct;168(4):578-85.
5. Pei YC, Fang TJ, Li HY, Wong AM. Cricothyroid muscle dysfunction impairs vocal fold vibration in unilateral vocal fold paralysis. Laryngoscope. 2014 Jan;124(1):201-6.
6. Liu KC, Lu YA, Lee LA, Li HY, Wong AM, Pei YC, et al. Cricothyroid muscle dysfunction affects aerodynamic performance in patients with unilateral vocal fold paralysis. J Voice. 2021 Aug 20 [Epub]. https://doi.org/10.1016/j.jvoice.2021.07.002.
7. Shaw GY, Searl JP, Hoover LA. Diagnosis and treatment of unilateral cricothyroid muscle paralysis with a modified Isshiki type 4 thyroplasty. Otolaryngol Head Neck Surg. 1995 Dec;113(6):679-88.
8. Wong KP, Lang BH, Ng SH, Cheung CY, Chan CT, Lo CY. A prospective, assessor-blind evaluation of surgeon-performed transcutaneous laryngeal ultrasonography in vocal cord examination before and after thyroidectomy. Surgery. 2013 Dec;154(6):1158-65.
9. Kim DH, Lee J, Seo Y, Kim SW, Hwang SH. Perioperative transcutaneous laryngeal ultrasonography to assess vocal cord function in thyroid surgery. Am J Surg. 2022 May;223(5):893-9.
10. Patel A, Spychalski P, Aszkielowicz A, Mikaszewski B, Kobiela J. Transcutaneous laryngeal ultrasound for vocal cord paralysis assessment in patients undergoing thyroid and parathyroid surgery: a systematic review and meta-analysis. J Clin Med. 2021 Nov;10(22):5393.
11. Hu Q, Zhu SY, Luo F, Gao Y, Yang XY. High-frequency sonographic measurements of true and false vocal cords. J Ultrasound Med. 2010 Jul;29(7):1023-30.
12. Cho W, Hong J, Park H. Real-time ultrasonographic assessment of true vocal fold length in professional singers. J Voice. 2012 Nov;26(6):819.
13. Hsiao TY, Wang CL, Chen CN, Hsieh FJ, Shau YW. Elasticity of human vocal folds measured in vivo using color Doppler imaging. Ultrasound Med Biol. 2002 Sep;28(9):1145-52.
14. Fang TJ, Pei YC, Hsin LJ, Lin WN, Lee LA, Li HY, et al. Quantitative laryngeal electromyography assessment of cricothyroid function in patients with unilateral vocal fold paralysis. Laryngoscope. 2015 Nov;125(11):2530-5.
15. Campbell MJ. Statistics at square one. 12th ed. Wiley-Blackwell; 2021.
16. Lammers MJ, Lea J, Westerberg BD. Guidance for otolaryngology health care workers performing aerosol generating medical procedures during the COVID-19 pandemic. J Otolaryngol Head Neck Surg. 2020 Jun;49(1):36.
17. Noel JE, Orloff LA, Sung K. Laryngeal evaluation during the COVID-19 pandemic: transcervical laryngeal ultrasonography. Otolaryngol Head Neck Surg. 2020 Jul;163(1):51-3.
18. Sciancalepore PI, Anzivino R, Petrone P, Petrone D, Quaranta N. Transcutaneous laryngeal ultrasonography: a promising tool for otolaryngologists during COVID-19. Am J Otolaryngol. 2021 JanFeb;42(1):102772.
19. Moisik SR, Lin H, Esling JH. A study of laryngeal gestures in Mandarin citation tones using simultaneous laryngoscopy and laryngeal ultrasound (SLLUS). J Int Phon Assoc. 2014 Apr;44(1):21-58.
20. Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985). 2014 Feb;116(3):291-301.
22. Woodson GE. Configuration of the glottis in laryngeal paralysis. I: clinical study. Laryngoscope. 1993 Nov;103(11 Pt 1):1227-34.
23. Wang CP, Chen TC, Yang TL, Chen CN, Lin CF, Lou PJ, et al. Transcutaneous ultrasound for evaluation of vocal fold movement in patients with thyroid disease. Eur J Radiol. 2012 Mar;81(3):e288-91.
24. Raghavendra BN, Horii SC, Reede DL, Rumancik WM, Persky M, Bergeron T. Sonographic anatomy of the larynx, with particular reference to the vocal cords. J Ultrasound Med. 1987 May;6(5):225-30.
25. Ooi LL, Chan HS, Soo KC. Color Doppler imaging for vocal cord palsy. Head Neck. 1995 Jan-Feb;17(1):20-3.
26. Hsiao TY, Wang CL, Chen CN, Hsieh FJ, Shau YW. Noninvasive assessment of laryngeal phonation function using color Doppler ultrasound imaging. Ultrasound Med Biol. 2001 Aug;27(8):1035-40.
27. Shau YW, Wang CL, Hsieh FJ, Hsiao TY. Noninvasive assessment of vocal fold mucosal wave velocity using color doppler imaging. Ultrasound Med Biol. 2001 Nov;27(11):1451-60.
28. Koufman JA, Walker FO, Joharji GM. The cricothyroid muscle does not influence vocal fold position in laryngeal paralysis. Laryngoscope. 1995 Apr;105(4 Pt 1):368-72.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







