 |
 |
- Search
AbstractObjectives. The study aimed to assess the relationship of tinnitus with hyperacusis with cognitive impairment as indicated by the Montreal Cognitive Assessment (MoCA) tool.
Methods. This multicenter cross-sectional study included individuals with chronic tinnitus from the “Unification of Treatments and Interventions for Tinnitus Patients” (UNITI) database. Participants were recruited from four different tertiary clinical centers located in Athens and Granada (Mediterranean group), as well as Berlin and Regensburg (German group). In total, 380 individuals with a diagnosis of non-pulsatile chronic tinnitus (permanent and constant tinnitus lasting more than 6 months) and no evidence of severe cognitive impairment (MoCA score >22) were enrolled. The evaluation utilized the following tools: MoCA, Tinnitus Handicap Inventory (THI), Hyperacusis Questionnaire (GÜF), Patient Health Questionnaire (PHQ-9), and the European School for Interdisciplinary Tinnitus Research Screening Questionnaire.
Results. MoCA scores differed between German and Mediterranean individuals (P<0.01), necessitating separate analyses for each group. In both cohorts, MoCA scores were significantly associated with education level, age, hearing threshold at 8 kHz, and THI. Furthermore, a significant correlation was observed between PHQ-9 scores and both THI and GÜF (P<0.01 for both Germans and those from the Mediterranean).
Tinnitus is a symptom defined by the conscious perception of a phantom, non-verbal tonal or composite sound in the absence of any external stimuli [1]. Tinnitus can appear as a symptom associated with other common conditions, including hearing loss (HL), anxiety, depression, or migraine [2]. More than 90% of patients with tinnitus show some form of HL [3], with the most common being high-frequency sensorineural hearing loss (SNHL). This type of hearing loss is typically observed in age-related HL or noise-induced HL [3]. Additionally, tinnitus can be associated with various otological diseases, including otosclerosis, vestibular schwannoma, or Meniere’s disease [1,3-5].
The term “tinnitus disorder” has been proposed to describe tinnitus that is associated with emotional distress, cognitive dysfunction, and/or autonomic arousal, which leads to behavioral changes and functional disability [6]. Given the impact of comorbidities on tinnitus, a screening tool has been developed to personalize the diagnosis and treatment of patients with tinnitus [1]. With this aim, the European School for Interdisciplinary Tinnitus Research (ESIT, https://esit.tinnitusresearch.net) [7] designed and validated the ESIT screening questionnaire (ESIT-SQ) [8]. Although this instrument has facilitated a standardized assessment of patients with chronic tinnitus [4], it does not evaluate cognition.
Several cross-sectional studies have consistently demonstrated an association between cognitive decline and HL across different populations [9]. Indeed, HL appears to exacerbate cognitive deficits in the elderly and may serve as a prognostic factor for mild cognitive impairment (MCI) [10]. Moreover, prospective cohort studies have suggested that the use of hearing aids in patients with HL can reduce cognitive decline in older adults [9]. Additionally, tinnitus-associated dysfunctional cognition, including “catastrophic thinking” and “avoidance cognition,” is strongly correlated with measures of tinnitus distress, anxiety, and depression. These cognitive patterns have been associated with impairment in executive function, attention, and memory [11]. Even mild cognitive symptoms can have a substantial impact on depression [11,12]. Furthermore, the prevalence of depression among individuals with severe impairment due to tinnitus sufferers has been reported to be as high as 60%–80% [13].
Given the association of tinnitus and age-related HL in elderly patients with cognitive impairment, and the increasing prevalence of dementia, including Alzheimer disease, in the elderly population, it may be useful to proactively diagnose HL and tinnitus in middle-aged adults. Furthermore, assessing cognitive impairment is of major clinical importance and may improve the characterization of patients with tinnitus and HL [14]. The aims of this study were to assess cognitive function in a non-selected cohort of patients with chronic tinnitus using the Montreal Cognitive Assessment (MoCA) questionnaire and to investigate which aspects of tinnitus, its comorbidities, and demographic variables are relevant for the extent of cognitive decline.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Granada (No. 1537-N-20). Written informed consent was obtained from all subjects involved in the study.
A total of 380 individuals with a diagnosis of non-pulsatile chronic tinnitus and no evidence of cognitive impairment (MoCA >22) were enrolled in a multi-center study. This cutoff point was established according to population studies, reporting that more than 60% of individuals obtained a score under 26 [11,15,16]. Tinnitus was defined as a permanent and constant tinnitus of at least 6 months of duration. Ninety-nine individuals were included from Berlin and 97 from Regensburg (Germany), 93 from Athens (Greece) and 91 patients from Granada (Spain) according to the inclusion and exclusion criteria published at the “Unification of Treatments and interventions for Tinnitus Patients” (UNITI) protocol in the UNITI randomized clinical trial (UNITIRCT) [11]. Clinical and psychometric variables were obtained according to the UNITI-protocol [11].
Individuals underwent an assessment of audiological symptoms and pure tone hearing thresholds, and a neuropsychological screening based on the MoCA for cognition, the Patient Health Questionnaire (PHQ-9) for depression [12,17], the Tinnitus Handicap Inventory (THI) and the Hyperacusis Questionnaire (GÜF) for tinnitus and hyperacusis, respectively [18,19]. The ESIT-SQ was used to collect information on demographics, lifestyle and general medical and otological history [8]. The study was part of the UNITI-project [11] and used data gathered over the course of the UNITI-RCT.
Standard air and bone conduction hearing thresholds were obtained by using an audiometer (AC-40, Interacoustics) in a soundproof booth (C-120, Diatec). The air-conduction hearing thresholds for the frequencies of 500 Hz, 1 kHz, and 2 kHz were retrieved from the audiograms and used to calculate the pure tone average (PTA) on each individual. The air conduction hearing thresholds at 4 kHz and 8 kHz were used as predictors of noiseinduced HL and age-related HL, respectively.
Individuals were categorized according to audiometric criteria defined in the UNITI-RCT protocol [11], distinguishing between bilateral SNHL (hearing thresholds >25 dB HL in both ears for any frequency from 0.5–4 kHz); unilateral SNHL (hearing thresholds >25 dB HL in one ear for any frequency from 0.5–4 kHz), or normal hearing (hearing thresholds ≤25 dB HL in both ears at any frequency from 0.5–4 kHz).
The MoCA test was used for the assessment of cognitive functions. The MoCA test covers working memory, short-term memory, linguistic functions, visuospatial capability or time and space orientation [12]. The scores obtained can range between 0 and 30. A cut-off point of 26 yielded the best balance between sensitivity and specificity for detecting MCI, according to the MoCA reference scores (https://mocacognition.com/). The following ranges may be used to grade severity: 18–25, MCI (average MoCA score: 22); 10–17, moderate cognitive impairment and Alzheimer diseases (average MoCA score: 16); <10, severe cognitive impairment. This test includes a score correction according to the patient’s academic level [20].
The PHQ-9 was used to assess depressive symptoms [21]. The PHQ-9 is a self-administered test, which consists of nine items assessing depressive symptoms, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria. The options for each question are “never” (0 points), “some days” (1 point), “more than half of the days” (2 points) and “almost every day” (3 points). The sum of the scores can range from 0 to 27 points (1–4, no depressive symptoms; 5–9, mild-depressive symptoms; 10–14, moderate-depressive symptoms; 15–27, severe-depressive symptoms) [17,21].
To assess the annoyance related to tinnitus, the THI was used. This questionnaire has 25 and 3 subscales: functional, emotional and catastrophizing tinnitus. Total scores range from 0 to 100 points, with 5 levels of severity: very mild (0–16), mild (18–36), moderate (38–56), severe (58–76), and catastrophic (78–100) [18,22-24].
This questionnaire consists of 15 questions and the total score obtained can range from 0 to 45 points. These scores can be organized into four handicap levels: grade I (score 0–10, slight handicap), grade II (11–17, moderate handicap), grade III (18–25, severe handicap), grade IV (26–45, very-severe handicap) [19].
ESIT-SQ is a self-administered questionnaire including 39 questions for clinical and tinnitus profiling: 17 general questions and 22 tinnitus-specific questions. It was developed with specific attention to questions about potential risk factors for tinnitus and tinnitus characteristics (including perceptual characteristics, modulating factors, and associations with co-existing conditions) [8].
The following demographic variables were retrieved from the ESIT-SQ: sex, age, educational level, episodes of vertigo and tinnitus-laterality. First, we performed a descriptive analysis for the MoCA, PHQ-9, THI, and GÜF scores (mean±standard deviation). The Shapiro-Wilk test was used to determine if the MoCA scores were normally distributed. Since MoCA scores did not follow a normal distribution, we used the nonparametric Mann-Whitney U-test to compare the MoCA scores across different centers and according to the hearing thresholds. Next, Spearman’s correlation coefficients were calculated to generate correlation matrices between all the variables, we performed a multiple lineal regression to estimate the effect of all variables in MoCA score. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using R-studio software (ver. 4.1.3, R Foundation for Statistical Computing).
We also tested several machine learning models to predict MoCA results (score <26 vs. ≥26) based on audiological and psychometric variables. For this, individuals were randomly allocated into two groups: training (80%) and test groups (20%) to compare four supervised machine learning algorithms: Logistic [25], eXtreme Gradient Boosting (XGBoost) [26], Adaptive boosting (AdaBoost) [27], and Gradient Boosted Decision Tree [28].
The distribution of MoCA scores was significantly different among participants recruited from the four centers (Fig. 1). There were no differences between individuals recruited in Berlin and Regensburg (P=0.54) or Athens and Granada (P=0.29); however, when we compared the MoCA scores between German and Mediterranean (MED) individuals, a statistically significant difference was found, with lower values for the MED subjects (P< 0.01). For this reason, we performed all statistical analyses separately for both groups.
A descriptive analysis of the main sociodemographic, audiological, and psychometric variables was performed (Table 1). The two groups (MED, n=184; and German, n=196) showed significant differences in age (MED, 50.2±12.2 years; German, 53.8± 12.8 years), hearing thresholds (PTA for MED, 24.77±14.17 dB; PTA for German, 16.01±10.49 dB), mean value of MoCA scores (MED, 26.11±2.22; German, 27.07±1.84), percentage of individuals with a MoCA score under of the cut-off (MED, 42.4%; German, 24.4%) and educational level, where the greatest difference was found in individuals with middle education level (MED, 9.3%; German, 33%), and university level (MED, 58.5%; German, 41.8%; all P<0.01).
Both groups were stratified according to hearing threshold, and individuals with bilateral SNHL were the group with greatest differences in age (MED, 55.22±10.28 years; German, 59.19± 10.04 years; P<0.01), PTA (MED, 32.11±14.15 dB; German, 21.08±10.15 dB; P<0.01), MoCA score (MED, 25.83±2.15; German, 27.03±1.86; P<0.01), percentage of patients with a MoCA score under the cut-off (MED, 45%; German, 22.4%; P<0.01), and educational level (MED, 14%; German, 34.8%; P<0.01).
For the unilateral SNHL group (MED, n=29; German, n=24), we also observed significant differences in PTA and educational level. Individuals with tinnitus and normal hearing (MED, n=55; German, n=56) also showed significant differences in PTA (MED, 13.43±4.48 dB; German, 6.84±4.50 dB; P<0.01), the percentage of patients with a MoCA score below the cut-off (MED, 40%; German, 16.1%; P<0.01), and level of education (MED, 3.6%; German, 27.3%; P<0.01).
MoCA scores were compared between individuals with normal hearing, unilateral HL, or bilateral HL, but no significant differences were found either in the MED group or the German group (Supplementary Tables 1 and 2).
To visualize the relationship among high-frequency HL, MoCA scores, and THI scores, 3D-scatter plots were designed for the MED and German groups (Fig. 2). Next, correlation matrices were calculated for all variables in the MED (Table 2) and German groups (Table 3). For the MED group, MoCA scores were associated with educational level (r=0.24, P<0.01), age (r=–0.19, P<0.01), THI scores (r=–0.15, P<0.05), and hearing thresholds in PTA (r=–0.16, P<0.05), at 4 kHz (r=–0.16, P<0.05), and 8 kHz (r=–0.15, P<0.05). For the German group, we observed statistically significant associations between MoCA scores and educational level (r=0.26, P<0.01), age (r=–0.25, P<0.01), THI scores (r=–0.22, P<0.01), the hearing threshold at 8 kHz (r=–0.18, P<0.05), and GÜF scores (r=0.15, P<0.05).
A multiple linear regression model was generated to predict MoCA scores according to THI scores, age, and hearing threshold in PTA, at 4 kHz, and at 8 kHz. In the German group, the model explained a moderate proportion of variance (F (5, 190)=5.80, P<0.001, adjusted R2=0.11); however, for the MED group, the model explained a small proportion of variance (F (5, 177)=3.10, P=0.010, adjusted R2=0.05).
Since 70% of individuals with tinnitus had bilateral or unilateral SNHL, we analyzed the impact on cognition of the covariates associated with the MoCA score (Table 4). We observed a significant effect of THI in both the MED (P<0.05), and German groups (P<0.01) and a significant effect of age in both groups (P<0.05), with a stronger effect in the German group (P<0.01).
No correlation was observed between PHQ-9 and MoCA scores either in MED (r=–0.14, P>0.05) or German participants (r=–0.10, P>0.05). However, significant associations were found between PHQ-9 and THI scores, and also for PHQ-9 and GÜF scores in both samples (all P<0.01).
The XGBoost algorithm was the method that best predicted cognitive decline in both groups. In the MED group, the variables age, GÜF score, hearing threshold at 8 kHz, THI score, and educational levels were used as predictors, and XGBoost showed an accuracy of 0.65 (95% confidence interval [CI], 0.47–0.80), with precision and recall of 0.7. In the German group, the model used the variables THI score, age, hearing threshold at 4 kHz, hearing threshold at 8 kHz and educational level as predictors, and XGBoost showed an accuracy of 0.76 (95% CI, 0.59–0.88), with a precision of 0.84 and a 0.80 recall.
Our results suggest an association between tinnitus distress and MCI. Higher THI scores were associated with lower MoCA scores. Although additional factors such as education level, age and high-frequency HL need to be considered in each individual, from a clinical perspective it seems reasonable to perform a cognitive screening in individuals with severe or catastrophic scores in the THI. Additionally, PHQ-9 scores were associated with tinnitus and hyperacusis handicap, regardless of the HL. These findings were found in two sets of participants recruited within the UNITI-RCT: MED (Athens and Granada) and German (Berlin and Regensburg).
An unexpected finding was the significant differences in the patient samples from Germany and the MED countries. Compared to the German cohort, the MED group had lower MoCA scores and higher PTA thresholds in the bilateral SNHL, unilateral SNHL, and normal hearing subgroups. The association between higher HL and lower cognitive function is known from individuals with Alzheimer disease, who have worse hearing thresholds in PTA than age-matched controls [29]. Our findings support an association of MoCA with the education level, age, hearing threshold at 8 kHz, and THI scores and confirm previous studies reporting the association of high-frequency HL and cognitive impairment [30,31].
Presbycusis is defined as bilateral, symmetric, progressive HL that increases with age, typically involving first the high frequencies [32]. In both groups, we found an association between highfrequency HL (8 kHz) and lower MoCA scores. A moderate association between moderate/severe HL and memory performance has been previously reported [32]. Other studies also reported that people with HL had significantly lower MoCA scores, than the normal-hearing population, and this demonstrated a clinically significant effect [31].
We found an association between lower MoCA scores and a worse hearing threshold at 4 kHz in the MED group. HL at 4 kHz has been related to noise exposure [33,34]. In this regard, a study in rodents reported a subset of younger animals with deficits in spatial learning after noise exposure. These findings suggest that noise exposure may be associated with an increased risk of cognitive impairment in spatial memory in vulnerable individuals [35].
This association between HL and cognitive impairment is consistent with the current literature, which confirms that hearing impairment is an independent and modifiable risk factor for cognitive impairment. Thus, it is necessary to understand the mechanisms underlying the correlation between hearing and cognition, in order to prevent the onset of hearing impairment and, therefore, cognitive impairment [36]. The differences found in hearing thresholds between the German and MED groups may explain the discrepancy in the MoCA store, although a larger sample size will be needed to confirm these findings. Research suggests that the prevalence of Alzheimer disease in Southern Europe is higher than in Northern Europe [37]. In our study, the MoCA scores were higher in the German group than in the MED group, and the percentage of individuals with MoCA scores below the cut-off was also lower in the German group (German, 24%; MED, 42.4%). Moreover, the hearing thresholds were significantly better in the German group than in the MED group, for all participants, as well as for individuals with bilateral SNHL or unilateral SNHL when analyzed separately.
We found a negative correlation between MoCA scores and THI scores in both groups. These results confirm that the prefrontal region governs cognitive functions [9,38], and frontal neural dysfunction may hinder tinnitus habituation and trigger emotional distress linked to the limbic system [39]. Moreover, patients with chronic tinnitus exhibited difficulties in attention and memory tests. According to the cognitive-perceptual load theory [40], the continuous perception of tinnitus may consume perceptual resources such as stimulus discrimination, contributing to an increased cognitive load [39,40]. In this sense. working memory issues could be more related to the emotional distress caused by tinnitus than to a direct impairment [41].
From our data, we can conclude that cognitive impairment in tinnitus patients is related to high THI scores, which in turn reflect the emotional distress induced by tinnitus [41]. As we have no data from a control group without tinnitus, we cannot draw any conclusions about a possible relationship between the perception of tinnitus and cognitive impairment. Moreover, numerous variables, such as age or HL, are directly associated with tinnitus and show correlations with cognitive function [42].
In our study, no significant correlation was found between PHQ-9 scores and MoCA scores. These results differ from the literature, where major depression is often associated with MCI [43]. Nevertheless, a significant correlation has been found between PHQ-9, THI scores, and GÜF scores, suggesting that hyperacusis is a relevant factor in severe tinnitus [44]. A systematic review including 18 studies between 1982 and 2011 found a significant association between tinnitus and depression (P<0.01) [45]. The authors postulated that there may be at least three potential connections between depression and tinnitus: “depression affecting tinnitus, tinnitus predisposing to depression, and tinnitus appearing as a comorbidity in patients with depression.” The majority of these studies found that depression either predisposes to tinnitus or occurs as a consequence of this symptom [44]. Given the strong association of tinnitus with anxiety, depression, HL and hyperacusis, we should use a wide range of instruments to assess the full impact of tinnitus on the patient’s quality of life, and we should consider referring patients for the treatment of depression, especially for patients who present with severe anxiety and hyperacusis [46]. There is an association between tinnitus-distress severity and cognitive function, which could be a critical element in the process of characterizing the psychological impact of tinnitus on each patient [14]. This is particularly relevant, as impaired cognitive abilities and attention deficits must be taken into account in individualized therapeutic management [47].
This study had several limitations. The most relevant is the lack of a control group that could be used as a reference for MoCA scores. However, most of the questionnaires included in this study were specifically symptom-oriented (e.g., THI for patients with tinnitus, GÜF for patients with hyperacusis); thus, the relevance of these questionnaires for healthy patients is unclear. In addition, the design of the UNITI-RCT, including audiological assessments and multiple questionnaires, prevented the use of other relevant tests for the detection of cognitive impairment, such as the Weschler intelligence test [48], Mini-Mental State Examination [49,50], or the Beck Depression Inventory-II [51,52].
▪ The Montreal Cognitive Assessment (MoCA) questionnaire is a screening tool suitable for detecting cognitive impairment in patients with tinnitus regardless of their hearing loss.
▪ MoCA scores suggest an association between tinnitus distress, high-frequency hearing loss and mild cognitive impairment.
▪ Patient Health Questionnaire (PHQ-9) scores were found to be associated with tinnitus and hyperacusis distress regardless of hearing loss.
NotesAUTHOR CONTRIBUTIONS Conceptualization: JALE, PPC. Formal analysis: ABR, PPC, JALE. Investigation: ABR. Methodology: ABR, PPC, JALE. Supervision: PPC, JALE. Writing–original draft: ABR, PPC, JALE. Writing–review and editing: all authors. ACKNOWLEDGMENTSABR is a PhD student in the Biomedicine Program at the School of Health Sciences at the University of Granada and this work is part of his doctoral thesis.
This study has been funded by the European Union’s Horizon 2020 Research and Innovation Programme, Grant Agreement Number 848261-H2020-SC1-BHC-2018-2020 (UNITI). PPC has received funds from the Andalusian Health Government (Grant RH-0150-2020).
Supplementary materialsSupplementary materials can be found online at https://doi.org/10.21053/ceo.2023.00808.
Supplementary Table 1.Correlation matrix for audiological, sociodemographic, and psychometric variables in Mediterranean individuals with chronic tinnitus and bilateral SNHL (n=100) Supplementary Table 2.Correlation matrix for audiological, sociodemographic, and psychometric variables in Mediterranean individuals with chronic tinnitus and unilateral SNHL (n=29) Fig. 1.Cognitive screening in individuals with chronic tinnitus. (A) Frequency histogram of the Montreal Cognitive Assessment (MoCA) scores from each site. (B) Boxplots of MoCA score differences according to hearing threshold in German and Mediterranean individuals. No differences are discernible. SNHL, sensorineural hearing loss. 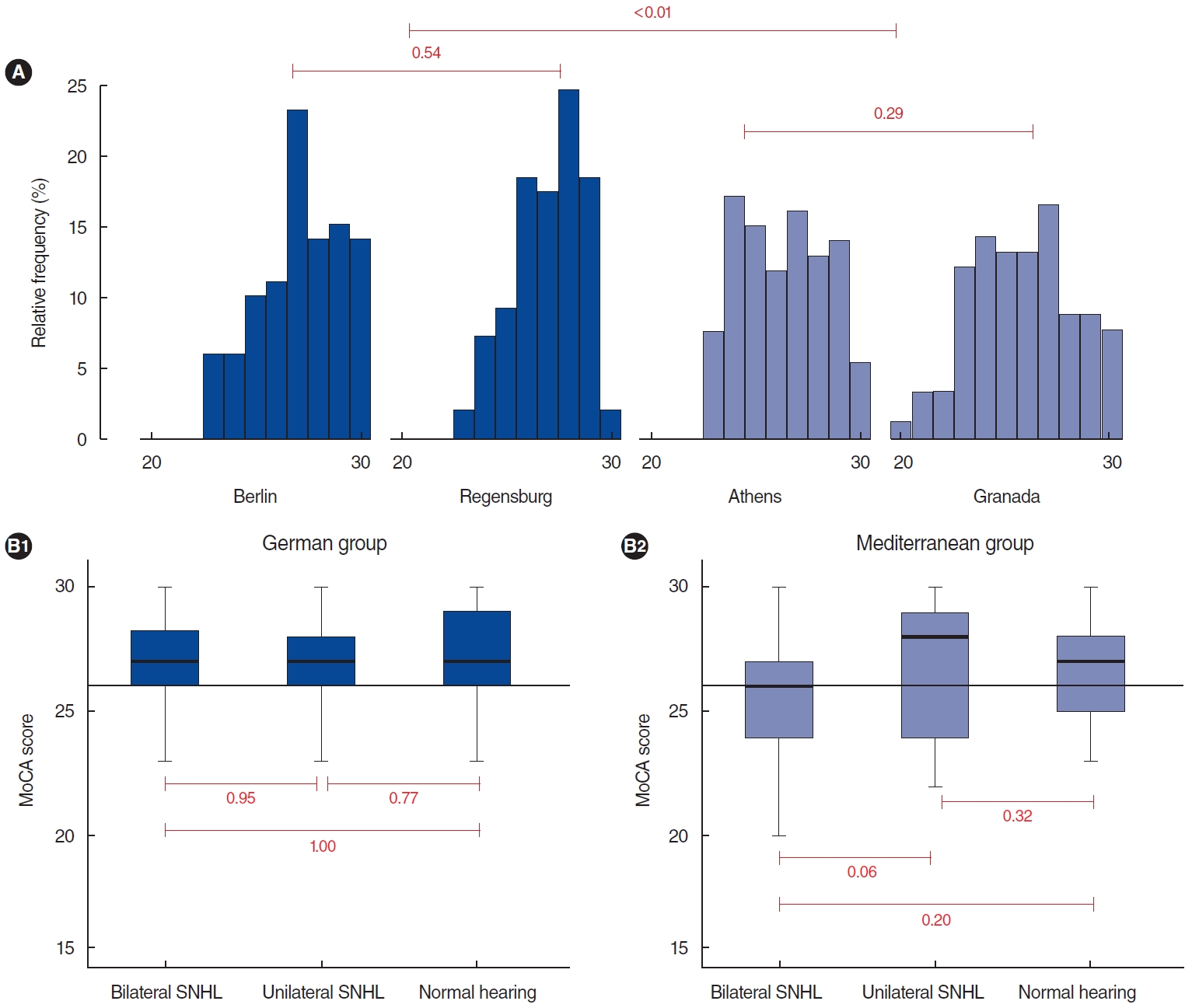
Fig. 2.Visualization of the relationship between Montreal Cognitive Assessment (MoCA) scores, Tinnitus Handicap Inventory (THI) scores, and average 8-kHz frequency values (high-frequency hearing loss). Variables in the German (A) and Mediterranean (B) groups in a three-dimensional scatterplot. 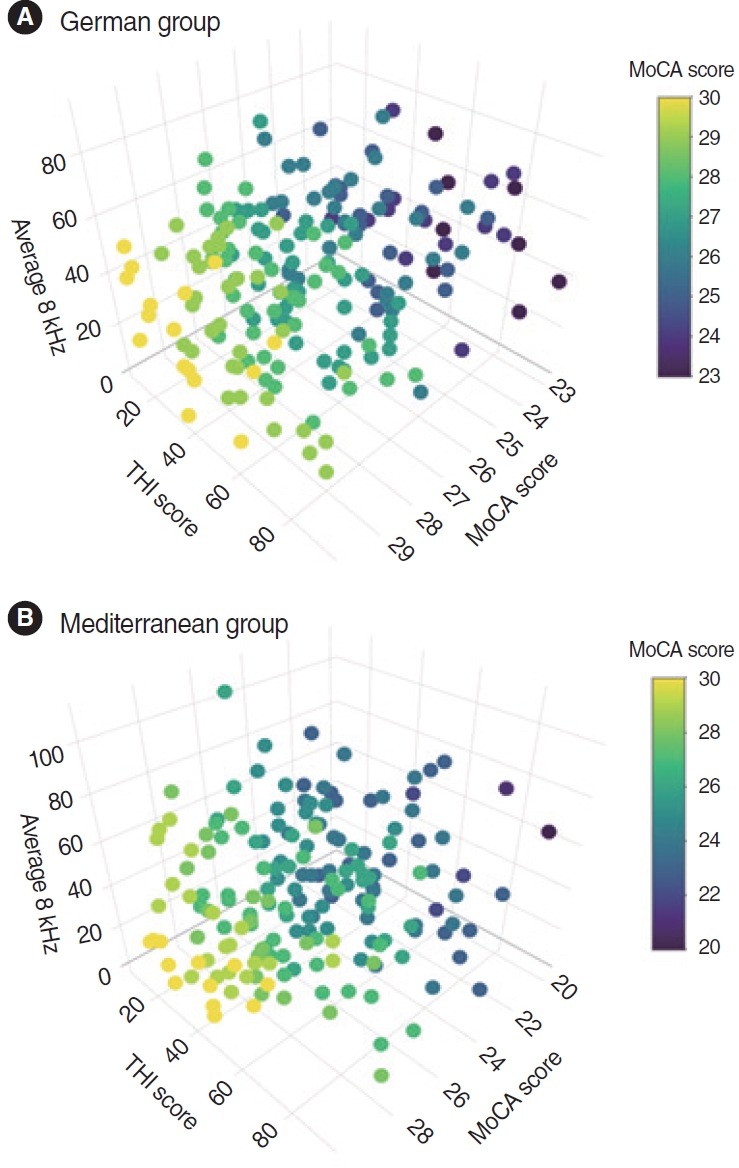
Table 1.Audiological and psychometric variables in Mediterranean and German individuals with chronic tinnitus (n=380)
Table 2.Correlation matrix for audiological, sociodemographic, and psychometric variables in Mediterranean individuals with chronic tinnitus (n=184)
Table 3.Correlation matrix for audiological, sociodemographic, and psychometric variables in German individuals with chronic tinnitus (n=196)
Table 4.Effects of covariates in multiple regression analysis for the Mediterranean and German groups
REFERENCES1. Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013 Sep;12(9):920-30.
2. Choi J, Lee CH, Kim SY. Association of tinnitus with depression in a normal hearing population. Medicina (Kaunas). 2021 Jan;57(2):114.
4. Haro-Hernandez E, Perez-Carpena P, Unnikrishnan V, Spiliopoulou M, Lopez-Escamez JA. Standardized clinical profiling in spanish patients with chronic tinnitus. J Clin Med. 2022 Feb;11(4):978.
5. Perez-Carpena P, Martinez-Martinez M, Martinez Carranza RA, Batuecas-Caletrio A, Lopez-Escamez JA. A tinnitus symphony in 100 patients with Meniere’s disease. Clin Otolaryngol. 2019 Nov;44(6):1176-80.
6. De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T, et al. Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res. 2021;260:1-25.
7. Schlee W, Hall DA, Canlon B, Cima RF, de Kleine E, Hauck F, et al. Innovations in doctoral training and research on tinnitus: the European School on Interdisciplinary Tinnitus Research (ESIT) Perspective. Front Aging Neurosci. 2018 Jan;9:447.
8. Genitsaridi E, Partyka M, Gallus S, Lopez-Escamez JA, Schecklmann M, Mielczarek M, et al. Standardised profiling for tinnitus research: the European School for Interdisciplinary Tinnitus Research Screening Questionnaire (ESIT-SQ). Hear Res. 2019 Jun;377:353-9.
9. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019 Dec;56:100963.
10. Malesci R, Brigato F, Di Cesare T, Del Vecchio V, Laria C, De Corso E, et al. Tinnitus and neuropsychological dysfunction in the elderly: a systematic review on possible links. J Clin Med. 2021 Apr;10(9):1881.
11. Schoisswohl S, Langguth B, Schecklmann M, Bernal-Robledano A, Boecking B, Cederroth CR, et al. Unification of Treatments and Interventions for Tinnitus Patients (UNITI): a study protocol for a multicenter randomized clinical trial. Trials. 2021 Dec;22(1):875.
12. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695-9.
13. Bhatt JM, Bhattacharyya N, Lin HW. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope. 2017 Feb;127(2):466-9.
14. Wang Y, Zhang JN, Hu W, Li JJ, Zhou JX, Zhang JP, et al. The characteristics of cognitive impairment in subjective chronic tinnitus. Brain Behav. 2018 Jan;8(3):e00918.
15. Nasreddine ZS, Phillips N, Chertkow H. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2012 Mar;78(10):765-6.
16. Melikyan ZA, Malek-Ahmadi M, O’Connor K, Atri A, Kawas CH, Corrada MM. Norms and equivalences for MoCA-30, MoCA-22, and MMSE in the oldest-old. Aging Clin Exp Res. 2021 Dec;33(12):3303-11.
17. Baader T, Molina JL, Venezian S, Rojas C, Farías R, Fierro-Freixenet C, et al. Validity and utility of PHQ9 (Patient Helth Questionnaire) in the diagnosis of depression in user patients of primary care in Chile. Rev Chil Neuropsiquiatr. 2012 Mar;50(1):10-22.
18. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996 Feb;122(2):143-8.
19. Herraiz C, de los Santos G, Diges I, Diez R, Aparicio JM. Assessment of hyperacusis: the self-rating questionnaire on hypersensitivity to sound. Acta Otorrinolaringol Esp. 2006 Aug-Sep;57(7):303-6.
20. Thomann AE, Goettel N, Monsch RJ, Berres M, Jahn T, Steiner LA, et al. The Montreal Cognitive Assessment: normative data from a German-speaking cohort and comparison with international normative samples. J Alzheimers Dis. 2018;64(2):643-55.
21. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the patient health questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001 Jul-Aug;63(4):679-86.
22. Herraiz C, Tapia MC, Plaza G. Tinnitus and Meniere’s disease: characteristics and prognosis in a tinnitus clinic sample. Eur Arch Otorhinolaryngol. 2006 Jun;263(6):504-9.
23. Herraiz C, Hernandez Calvin J, Plaza G, Tapia MC, de los Santos G. Disability evaluation in patients with tinnitus. Acta Otorrinolaringol Esp. 2001 Aug-Sep;52(6):534-8.
24. Kleinjung T, Fischer B, Langguth B, Sand PG, Hajak G, Dvorakova J, et al. Validation of the German-version Tinnitus Handicap Inventory (THI). Psychiat Prax. 2007 Jan;34(Supplement 1):S140-2.
25. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22.
26. Chen T. Guestrin C. XGBoost: a scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2016 Aug 13-17; San Francisco, CA. Association for Computing Machinery; 2016. p. 785-94.
27. Alfaro E, Gamez M, Garcia N. Adabag: an R package for classification with boosting and bagging. J Stat Softw. 2013 Sep;54:1-35.
28. Ye J, Chow JH, Chen J, Zheng Z. Stochastic gradient boosted distributed decision trees. In: Proceedings of the 18th ACM conference on Information and knowledge management; 2009 Nov 2; Hong Kong. Association for Computing Machinery; 2009. p. 2061-4.
29. Kwok SS, Nguyen XT, Wu DD, Mudar RA, Llano DA. Pure tone audiometry and hearing loss in Alzheimer’s disease: a meta-analysis. Front Psychol. 2022 Jan;12:788045.
30. Diao T, Ma X, Zhang J, Duan M, Yu L. The correlation between hearing loss, especially high-frequency hearing loss and cognitive decline among the elderly. Front Neurosci. 2021 Nov;15:750874.
31. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018 Feb;144(2):115-26.
32. Deal JA, Sharrett AR, Albert MS, Coresh J, Mosley TH, Knopman D, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. 2015 May;181(9):680-90.
33. Chang TY, Liu CS, Huang KH, Chen RY, Lai JS, Bao BY. High-frequency hearing loss, occupational noise exposure and hypertension: a cross-sectional study in male workers. Environ Health. 2011 Apr;10:35.
34. Pirila T. Left-right asymmetry in the human response to experimental noise exposure. I. Interaural correlation of the temporary threshold shift at 4 kHz frequency. Acta Otolaryngol. 1991;111(4):677-83.
35. Patel SV, DeCarlo CM, Book SA, Schormans AL, Whitehead SN, Allman BL, et al. Noise exposure in early adulthood causes age-dependent and brain region-specific impairments in cognitive function. Front Neurosci. 2022 Oct;16:1001686.
36. Bisogno A, Scarpa A, Di Girolamo S, De Luca P, Cassandro C, Viola P, et al. Hearing loss and cognitive impairment: epidemiology, common pathophysiological findings, and treatment considerations. Life (Basel). 2021 Oct;11(10):1102.
37. Niu H, Alvarez-Alvarez I, Guillen-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia. 2017 Oct;32(8):523-32.
38. Neff P, Simoes J, Psatha S, Nyamaa A, Boecking B, Rausch L, et al. The impact of tinnitus distress on cognition. Sci Rep. 2021 Jan;11(1):2243.
39. Mazurek B, Bocking B, Dobel C, Rose M, Bruggemann P. Tinnitus and influencing comorbidities. Laryngorhinootologie. 2023 May;102(S 01):S50-8.
40. Khan RA, Husain FT. Tinnitus and cognition: can load theory help us refine our understanding. Laryngoscope Investig Otolaryngol. 2020 Nov;5(6):1197-204.
41. Waechter S. Association between hearing status and tinnitus distress. Acta Otolaryngol. 2021 Apr;141(4):381-5.
42. Nagaraj MK, Bhaskar A, Prabhu P. Assessment of auditory working memory in normal hearing adults with tinnitus. Eur Arch Otorhinolaryngol. 2020 Jan;277(1):47-54.
43. Culpepper L, Lam RW, McIntyre RS. Cognitive impairment in patients with depression: awareness, assessment, and management. J Clin Psychiatry. 2017 Nov/Dec;78(9):1383-94.
44. Cederroth CR, Lugo A, Edvall NK, Lazar A, Lopez-Escamez JA, Bulla J, et al. Association between hyperacusis and tinnitus. J Clin Med. 2020 Jul;9(8):2412.
45. Geocze L, Mucci S, Abranches DC, Marco MA, Penido Nde O. Systematic review on the evidences of an association between tinnitus and depression. Braz J Otorhinolaryngol. 2013 Jan-Feb;79(1):106-11.
46. Aazh H, Moore BC. Factors associated with depression in patients with tinnitus and hyperacusis. Am J Audiol. 2017 Dec;26(4):562-9.
47. Sherlock LP, Brungart DS. Functional impact of bothersome tinnitus on cognitive test performance. Int J Audiol. 2021 Dec;60(12):1000-8.
48. Cristofani P, Di Lieto MC, Casalini C, Pecini C, Baroncini M, Pessina O, et al. Specific learning disabilities and emotional-behavioral difficulties: phenotypes and role of the cognitive profile. J Clin Med. 2023 Feb;12(5):1882.
49. Papatsimpas V, Vrouva S, Papadopoulou M, Papathanasiou G, Bakalidou D. The effects of aerobic and resistance exercises on the cognitive and physical function of persons with mild dementia: a randomized controlled trial protocol. Healthcare (Basel). 2023 Feb;11(5):677.
50. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189-98.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||







