Neurocognition of Aged Patients With Chronic Tinnitus: Focus on Mild Cognitive Impairment
Article information
Abstract
Objectives.
To investigate the neurocognition of aged patients with chronic tinnitus and reveal the possible association between tinnitus severity and cognitive function, with attention to mild cognitive impairment (MCI).
Methods.
Fifty-eight elderly patients (≥65 years old) with chronic tinnitus (≥6 months) were prospectively enrolled in this study. All patients assessed the neurocognitive batteries including the Korean version of the patient health questionnaire-9 (K-PHQ-9), the Lawton instrumental activities of daily living scale (K-IADL), and the Montreal cognitive assessment (MoCA-K). After initial evaluation to exclude moderate or severe cognitive impairment by a psychiatrist, the patients were classified into two groups: MCI and non-MCI, according to the MoCA-K scores (cutoff value, 22/23). All patients underwent audiological examinations including psychoacoustic tests of tinnitus.
Results.
Of 58 patients, 10 (17.2%) met the MCI criteria. The tinnitus handicap inventory (THI) score in the MCI group was significantly higher than that in the non-MCI group. Based on multivariate regression analysis, a significant association between tinnitus severity and MoCA-K score was also detected. Specifically, bothersome tinnitus (THI score ≥30) was closely linked to the presence of MCI. Meanwhile, the impact of MCI on both K-PHQ-9 and K-IADL scores was not evident in patients with chronic tinnitus.
Conclusion.
Tinnitus severity appears to be a potential independent determinant for predicting the MCI, suggesting the underlying mechanism between chronic tinnitus and cognitive deficit. Given that MCI highly links to dementia, the evaluation of cognitive functions in aged patients with chronic tinnitus need to be considered at the initial assessment of tinnitus.
INTRODUCTION
Non-pulsatile tinnitus is a common otologic symptom, characterized by conscious auditory perception in the absence of an external stimulus; this is often called phantom sound as there is no corresponding genuine physical source of the sound [1]. Although the exact pathophysiology remains elusive, phantom sound has been postulated to be caused by maladaptive cortical neural plasticity and resulting hyperactivity that reflects a complex neural network interplay between auditory and non-auditory cortices [2-4]. The constant awareness of this phantom auditory perception often causes considerable distress. In addition to psychological complications, such as social and emotional difficulties, previous studies have also reported cognitive impairment in patients with chronic tinnitus [5-8].
Cognitive function typically involves attention, concentration, use of working memory and information processing [6]. Several studies have evaluated the association between cognitive impairment and chronic tinnitus; tinnitus patients showed poor performance in various cognitive tasks involving attention and working memory [9-11]. A significant correlation between cognitive processing speed and tinnitus handicap inventory (THI) was found in patients with bothersome tinnitus (THI score ≥30) [12]. Recently, electroencephalography (EEG) revealed that neural changes in the hippocampus, anterior cingulate, and insula were associated with cognitive deficits in patients with chronic tinnitus [13]. Hence, neurophysiological changes in patients with tinnitus might be affected by the nature of cognitive deficits.
Despite the existence of putative evidence between tinnitus and cognitive deficits, only a few published studies have yet explored the cognitive function of chronic tinnitus patients [11, 12,14,15]. Notably, there was no study that suggested the possible association between chronic tinnitus and mild cognitive impairment (MCI). MCI is a clinical syndrome thought to represent the intermediate cognitive state between normal aging and dementia. Patients aged ≥65 years diagnosed with MCI are more likely to develop dementia than normal elderly people [16]. When patients are diagnosed with MCI, nondisabled level of cognitive decline in their activities of daily living or instrumental activities of daily living are maintained. However, their cognitive abilities tend to deteriorate at a rate in-between that of normal elderly people and mild Alzheimer disease patients, resulting in a higher risk of dementia [17]. Hence, evaluating the presence of MCI in patients with chronic tinnitus may clarify these issues. Thus, we aimed to investigate the cognitive and psychological characteristics of aged patients with chronic tinnitus and to analyze the causal link between tinnitus and MCI.
MATERIALS AND METHODS
Patients
From March 2017 to March 2018, consecutive chronic tinnitus (≥6 months) patients aged 65 years or more were prospectively enrolled. We excluded cases with (1) hearing loss >40 dB in at least one ear (calculated by averaging the pure-tone audiometry [PTA] thresholds at 0.5, 1, 2, and 4 kHz); (2) high-frequency hearing loss (increasing thresholds from 0.25 to 8 kHz and the difference between the thresholds at 250 and 8,000 Hz was >20 dB HL) [18]; (3) known history of neuropsychiatric or brain disorder; (4) current (within 3 months) psychotropic or central nervous system-active medications; (5) alcohol or drug abuse; (6) hearing aids or sound generators that may influence hearing and tinnitus level.
Finally, 58 eligible patients were enrolled in this study. All patients were assessed using the MoCA-K, a Korean version of the Montreal cognitive assessment designed to screen for MCI. After further excluding the moderate or severe cognitive impairment by a psychiatrist (JYL), patients were classified into the MCI group (n=10; MoCA-K score <23) or into the non-MCI group (n=48; MoCA-K score ≥23) based on a cutoff value of 22 [19]. This study was approved by the Institutional Review Board of the SMG-SNU Boramae Medical Center (IRB No. 16-2016-401), and patient consent was waived.
Audiological and psychoacoustic evaluations
At the initial visit, a structured history of the characteristics of tinnitus on the affected side and the psychoacoustic nature (pure-tone or narrow-band noise) of tinnitus was obtained. All subjects underwent PTA testing that included psychoacoustic tests of tinnitus, such as tinnitus pitch and loudness matching. The hearing thresholds for seven different octave frequencies (0.25, 0.5, 1, 2, 3, 4, and 8 kHz) were evaluated using PTA in a soundproof booth. The mean hearing threshold was calculated using the average of the hearing thresholds at 0.5, 1, 2, and 4 kHz. For analysis, we selected the hearing threshold in the tinnitus affected side. In cases of patients with bilateral tinnitus, we documented the mean hearing threshold in the dominant tinnitus side. The perceived tinnitus handicap was measured using THI. Based on a previous study, bothersome tinnitus was defined as THI ≥30 [12]. We also assessed the visual analog scale (VAS) scores (patients answering questions on four aspects of tinnitus such as loudness, annoyance, awareness, and effect of life during daytime on a scale from 0 to 10).
Neurocognitive evaluation
All patients underwent a battery of neurocognitive tests, including three questionnaires at initial assessment for tinnitus: Korean version of the patient health questionnaire-9 (K-PHQ-9), Korean version of the Lawton instrumental activities of daily living scale (K-IADL), and MoCA-K.
MoCA-K
MoCA-K is a Korean version of rapid screening neurocognitive test for MCI. It can be completed in 10 minutes. It scores from 0 to 30, with high scores indicating better cognition. Based on a previous study employing neuropsychological batteries for MCI screening, the MoCA-K displayed excellent sensitivity (89%) and good specificity (84%) with the implementation of the cutoff value of 22 [19]. Thus, we decided to define MCI as an MoCA-K score below 23. Attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculation, and orientation were assessed. In the MoCA-K, the words used in the short-term memory recall task and the letters used in the trail-making B task were changed, and the phonemic fluency task was replaced with a semantic fluency task. One point was added for participants with 6 or fewer years of education instead of 12 or fewer years of education. The other tests were similar to those in the original MoCA [19].
K-PHQ-9
PHQ was developed for the prompt diagnosis of neuropsychiatric disorders [20]. PHQ-9, a simple self-reporting assessment for depression screening, primarily focuses on depression severity measurement. The score ranges from 0 to 27, and the completion time is less than 3 minutes. PHQ-9 comprises of nine items and is used for depression diagnosis according to the diagnostic and statistical manual of mental disorders, fifth edition (DSM-IV). In this study, all patients underwent assessment using the K-PHQ-9. According to previous studies, the optimal cutoff score for major depression is <10 in PHQ-9, with 88.5% sensitivity and 94.7% specificity [21]. Moreover, K-PHQ-9 has been reported as a reliable and effective tool for screening depression [22].
K-IADL
The K-IADL, a Korean version of a self-reporting questionnaire to assess difficulties in performing instrumental activities of daily living, was given to all patients. K-IADL comprises of 11 items, with the score of each item ranging from 0 to 3 as follows: 0, normal; 1, with some assistance; 2, with much assistance; 3, unable to do; NA, not applicable. The K-IADL consists of eleven self-evaluating questions concerning capacity and difficulty in performing activities of daily living, including (1) shopping, (2) mode of transportation, (3) ability to handle finances, (4) housekeeping, (5) preparing food, (6) ability to use a telephone, (7) responsibility for own medication, (8) recent memory, (9) hobbies, (10) watching television, and (11) fixing things around the house. Based on a previous study, K-IADL showed excellent internal consistency and test-to-test reliability. K-IADL has been reported as a valid and reliable tool for evaluating functional status in the elderly, especially when using a cutoff of 10 in measuring daily living activities [23]. Moreover, K-IADL findings have been consistent with other general cognitive indices, demonstrating good validity [23].
Statistical analysis
All data were analyzed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). To determine significant differences between the two groups in the screened continuous and categorical variables, independent Student t-tests, Mann-Whitney U-test and chi-square tests were performed, as appropriate. To analyze the association between tinnitus severity (based on THI score) and cognitive deficits (based on MoCA-K score), Pearson correlation analysis was performed. Multivariate and logistic regression analysis was performed to simultaneously assess the relative influence of cognitive deficit and associated variables including the age, sex, duration of tinnitus, and mean hearing threshold. P-values <0.05 were considered to indicate statistical significance.
RESULTS
Demographic and clinical characteristics
The patients’ demographic and clinical characteristics are presented in Table 1. The mean age of the patients was 68.1±5.1 years (range, 65 to 82 years). The number of male and female was 23 (39.7%) and 35 (60.3%), respectively. The median duration of tinnitus was 2 years (range, 0.5 to 20 years). The rate of MCI based on MoCA-K was 10 out of 58 (17.2%). When comparing between the MCI and non-MCI groups, no significant difference in age (70.9±7.9 vs. 67.5±4.1 years, P=0.211), duration of tinnitus (P=0.731), or sex (P=0.287) was detected. There was a significant difference in hearing threshold (33.0±7.4 vs. 25.7± 8.7 dB, P=0.017) between two groups. But, the speech discrimination (91.6%±12.4% vs. 93.3%±7.2%, P=0.407) in the MCI group was similar to that in the non-MCI group. An individualized profile of each subject was presented in Supplementary Table 1.
Comparison of neurocognitive results
The mean MoCA-K, median K-PHQ-9, and median K-IADL scores from all patients were 24.5±4.0 (range, 8 to 29), 3 (range, 0 to 26), and 8 (range, 5 to 14), respectively. The MoCA-K score, as expected, was significantly different (18.9±3.2 vs. 26.1±1.8, P<0.001) between the MCI and non-MCI group. On the other hand, the K-IADL and K-PHQ-9 scores showed no significant change between the MCI group and non-MCI group (P=0.411 and P=0.261, respectively) (Fig. 1).
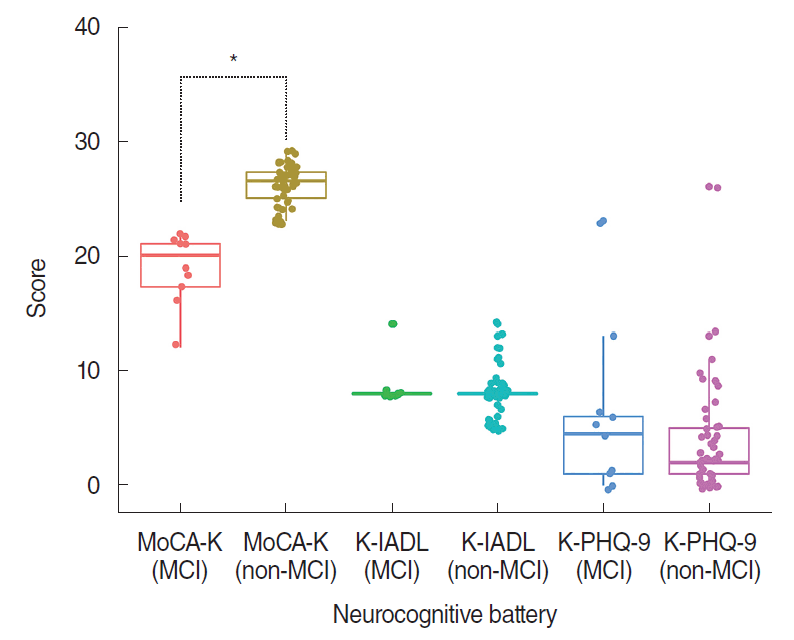
Comparison of neurocognitive test results based on the presence of mild cognitive impairment (MCI) in aged patients (≥65 years) with chronic tinnitus. In the MCI group, the Korean version of the Montreal cognitive assessment (MoCA-K) score was significantly lower than that in the non-MCI group (P<0.001). However, Korean version of the Lawton instrumental activities of daily living scale (K-IADL) and Korean version of the patient health questionnaire-9 (K-PHQ-9) scores were similar regardless of the presence of MCI (P=0.411 and P=0.261, respectively). *P<0.05 by independent t-test.
Characteristics with respect to tinnitus
The psychoacoustic characteristics (tinnitus pitch and loudness) of patients between the MCI group and the non-MCI group were similar (Table 2). Of the 58 patients, 21 (36.2%) were bothered by their tinnitus according to our set criteria (THI ≥30). Notably, the THI score of the MCI group was significantly higher than that of the non-MCI group (33.6±10.4 vs. 21.9±10.1, P=0.002). Moreover, there was a significant association between MCI (MoCA-K <23) and bothersome tinnitus (THI ≥30) but not in the patients who were not bothered by tinnitus (THI <30) (Table 3). VAS scores, including awareness (6.6±3.2 vs. 5.8±3.1, P=0.372), annoyance (5.4±2.9 vs. 4.0±2.9, P=0.109), loudness (5.5±2.6 vs. 4.4±2.6, P=0.177), and effect of life (3.2±2.4 vs. 3.0±2.9, P=0.77), were not statistically different between the MCI and non-MCI groups.
Association of tinnitus severity and cognitive function
Pearson coefficient analysis among 58 chronic tinnitus patients revealed a significant negative correlation between THI and MoCA-K scores (r=–0.837, P<0.001) (Fig. 2A). Based on multivariate regression analysis, an increase of THI was significantly associated with a decrease of MoCA-K score (estimate: –0.170; standard error, 0.032; P<0.001), after adjusting age, sex, duration of tinnitus, and mean hearing threshold. In line with this, based on logistic regression analysis, an increase of THI score was significantly associated with the presence of MCI (odds ratio [OR], 1.12; 95% CI, 1.02 to 1.23; P=0.014), after adjusting age, sex, duration of tinnitus, and mean hearing threshold. The significant predictors that contribute to the development of MCI were both mean hearing threshold (OR, 1.14) and tinnitus severity (OR, 1.13) (Fig. 2B).
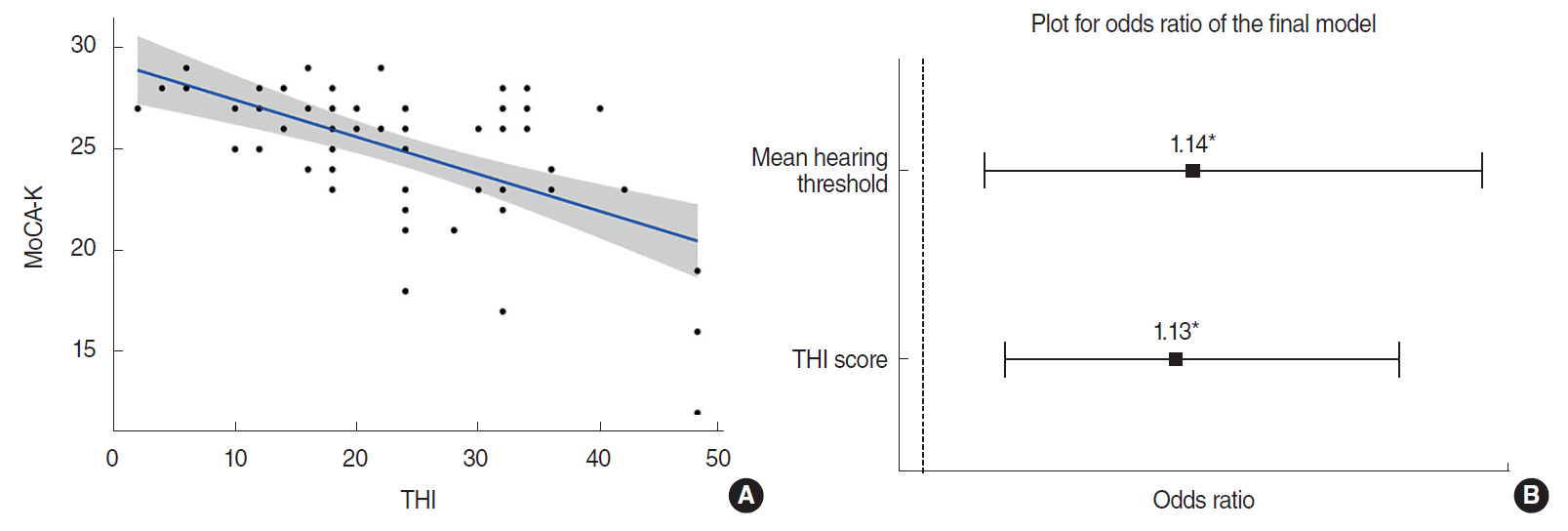
Association between tinnitus severity and cognitive function in aged patients (≥65 years) with chronic tinnitus. (A) A statistically significant negative correlation was detected between tinnitus handicap inventory (THI) and Korean version of the Montreal cognitive assessment (MoCA-K) scores in all patients (r=–0.837, P<0.001). (B) A plot for odds ratios of the final model. As a result of logistic regression analysis, either mean hearing threshold or THI scores significantly contributed to the developing mild cognitive impairment. *P<0.05 by logistic regression analysis.
DISCUSSION
The present study aimed to investigate the association between tinnitus severity and cognitive function, especially in patients with MCI, among aged patients with chronic tinnitus. Ten of 58 patients with chronic tinnitus (17.2%) were diagnosed with MCI based on the MoCA-K score (cutoff value, 22). The MCI incidence in this study is similar to that reported by a previous study (10%–20% among elderly patients aged >65 years) [24]. Based on logistic regression analysis, tinnitus severity is independently associated with the presence of MCI in aged patients with chronic tinnitus. More specifically, a significant association between the presence of MCI and bothersome tinnitus (THI ≥30) was found in the present study.
The impact of tinnitus severity on MCI presence
Several previous studies have indicated the presence of cognitive impairments, including attention deficit, instant memory disorder, and inefficient learning in patients with chronic tinnitus [6,15]. Despite inconsistent results on the association of tinnitus with certain types of cognitive functions, tinnitus has been shown to have negative effects on executive or selective attention and working memory [6]. A study suggested that the presence of chronic tinnitus may be associated with reduced cognitive functioning, particularly attention [25]. The cognitive deficit underlying chronic tinnitus may involve a failure in directing attention away from a phantom sound. Recently, a significant impairment in cognitive control and inhibition was detected in chronic tinnitus patients [13]. In this regard, it is yet to be validated whether chronic tinnitus may increase the risk of MCI by leading to neurocognitive network changes influencing attention-switching. Meanwhile, working memory has a limited capacity that are relevant theoretical composition for cognition [6]. Tinnitus could affect cognition such that it reduces the cognitive capacity available for conducting other tasks [11]. Additionally, both tinnitus and hearing could each separately elicit overload on the central executive [6], which may result in ineffective working memory. From this perspective, even though most patients involved in this study had normal hearing or mild hearing impairments, tinnitus may constitute in the development of MCI, especially in patients with severe tinnitus.
There is a growing body of evidence indicating that the severity of chronic tinnitus is associated with a broad range of cognitive performance. As shown previously, the more severe the tinnitus, the more likely the deterioration of cognitive processing is, suggesting that cognitive processing speed is an independent predictor of tinnitus severity [12]. Furthermore, a neuropsychological study reported a significant negative correlation between tinnitus loudness and duration, and performance on neurocognitive tasks [15]. In this regard, the increase in tinnitus severity may negatively affect attentional orientation and executive control, reducing cognitive processing speed [6]. Similarly, one study reported that patients with severe tinnitus presented with lower scores during cognitive ability screening assessments in almost all subdomains, as compared to mild or moderate tinnitus patients [14]. Moreover, tinnitus severity was negatively correlated with cognition and positively correlated with N2 and P3 latencies of the P300 event-related potential (ERP) [14]. Given that ERPs provide an objective evaluation of attention domains, an increase in tinnitus severity might adversely impact cognitive performance via auditory inattention, which may in turn potentiate cognitive deficits. Consistent with previous studies, the present study demonstrated an association between bothersome tinnitus (THI ≥30) and MCI development. Even though the presence of MCI did not negatively affect patients’ quality of life in this study, without intervention, severe tinnitus may be associated with serious cognitive deficits, such as dementia. Such deficits may in turn lead to a direct decrease in quality of life and work efficiency. However, this hypothesis awaits further confirmation.
The present study revealed that cognitive impairment is common in tinnitus patients. However, the exact pathophysiology behind the development of tinnitus-associated cognitive deficits has not been yet elucidated. A neuropsychological study investigating cognitive processing in chronic tinnitus patients revealed that tinnitus disrupts non-auditory processing of verbal, visual, and visual-spatial information, suggesting the development of neuroplastic changes [15]. Furthermore, a neuroimaging study employing EEG with source analysis recently reported the nature of the cognitive deficits in tinnitus patients [13]. Notably, a negative correlation was found between neural activity in the hippocampus, the anterior cingulate, and insula, and MoCA score [13]. A previous study also reported a negative correlation between changes in EEG activity in cognition-related regions and THI score after tinnitus treatment using a neurophysiological model [3]. The increased neural activity during the resting state in chronic tinnitus patients was correlated with the need for more effort in cognitive processing task completion [13]. Thereby, patients with severe tinnitus are highly likely to present with maladaptive cortical plasticity that may attenuate cognitive performance.
Implication of MCI screening in aged patients with chronic tinnitus
The evaluation of cognitive screening tests for patients with severe tinnitus may be beneficial for the appropriate management of both tinnitus and MCI. The impact of tinnitus on quality of life is better defined by identifying the associated cognitive impairments for optimal rehabilitation. Given the high risk of dementia in patients with MCI [26,27], cognitive function testing would allow clinicians to validate hindered cognitive difficulties that may progress to dementia, as well as track any changes in perceived cognitive difficulties and tinnitus distress over the course of treatment. In this study, patients with MCI appeared to experience more difficulties in daily life due to chronic tinnitus compared to patients with normal cognitive function. Taken together, chronic tinnitus may be a potential predictor of MCI that progresses to dementia; thereby, the evaluation of cognitive functions in aged patients with chronic tinnitus need to be considered at the initial assessment of tinnitus.
Limitations and future perspectives
To the best of our knowledge, this is the first study to examine the cognitive and psychologic aspects of aged patients with chronic tinnitus with a special focus on the presence of MCI, and its association with severe tinnitus. Although we demonstrated significant findings, there are several potential limitations that should be controlled in further investigations.
First, we addressed a correlation between tinnitus severity and MCI; however, we were unable to identify the causal link between the two because we did not evaluate the changes of cognitive function after tinnitus treatment. Further investigation of cognition change according to tinnitus improvement may be necessary to validate this causal association. Additionally, previous study showed that the existence of depression and/or anxiety negatively commonly underlie in tinnitus patients than in healthy controls [28]. In other word, tinnitus-related emotional state disorder has been closely linked to cognitive deficits [29]. Although we evaluated depression status and the activities of daily living using self-reporting questionnaires, variable emotional states might introduce bias and hinder the cognitive ability assessment due to the nature of cross-sectional study. Likewise, we assessed for MCI once during the initial screening. Considering that the cognition of approximately 15%–20% of MCI patients spontaneously improves without treatments after 1–2 years [26], longer follow-ups are needed to clearly demonstrate the association between tinnitus and MCI. Taken together, it is evident that a comprehensive neuropsychological investigation for chronic tinnitus towards highly complex neurobiological process is necessary.
Second, Lin et al. [30] demonstrated the hazard ratio (95% confidence interval) for incident all-cause dementia was 1.89 (1.00 to 3.58) for mild hearing loss, compared to normal hearing. Nonetheless, we included the patients with mild hearing impairment due to a severe loss of cohort when performing strict audiological criteria. Although the association of mild hearing loss and MCI remains unknown, our results might be biased by the confounder. Therefore, strict normal hearing criteria may be needed to prove what the authors claim. In a recent study, subjects with tinnitus and severe acquired hearing loss exhibited higher cortical activity in the parahippocampus than did non-tinnitus controls [31]. This increase may be associated with abnormally enhanced attention and impaired memory leading to cognitive deficits. Thus, absence of severe or profound hearing loss in our subjects might hinder the precise result interpretation. Therefore, future studies exploring the role of the degree of hearing in tinnitus-associated MCI should be considered.
Third, the present study did not adopt an objective approach such as neuroimaging or electrophysiology in the examination of the nature of these cognitive deficits. Neurobiological modelling studies have reported that brain areas like the limbic system, autonomic nervous system, and sensory cortex may contribute to the association between tinnitus and cognitive deficits [15,32]. Therefore, neurobiological and neuroimaging approaches should be implemented to aid with the identification of the cognitive impairment etiology in chronic tinnitus patients. At present, we are conducting the neurobiological study combined with neuroimaging to reveal the casual link between tinnitus and MCI.
Our results suggest that tinnitus severity was closely linked to cognitive deterioration that results in MCI. Therefore, evaluation of cognitive function in aged patients with chronic tinnitus needs to be considered at the initial assessment to allow for appropriate tinnitus and MCI management.
HIGHLIGHTS
▪ For the first time, we investigated the possible association between tinnitus and cognitive function, with attention to mild cognitive impairment (MCI).
▪ Of 58 elderly patients with chronic tinnitus, 10 (17.2%) met the MCI criteria (based on Korean version of the Montreal cognitive assessment [MoCA-K] <23).
▪ The tinnitus handicap inventory score in the MCI group was significantly higher than that in the non-MCI group.
▪ A significant negative correlation was found between tinnitus severity and MoCA-K score; given that MCI highly links to dementia, the evaluation of cognitive functions in aged patients with chronic tinnitus need to be considered at the initial assessment of tinnitus.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a clinical research grant provided by SMG-SNU Boramae Medical Center, Seoul, Republic of Korea.
Notes
AUTHOR CONTRIBUTION
Conceptualization: YHK. Data curation: YJS, SYH. Formal analysis: SYL. Methodology: SYL, JYL, YHK. Project administration: JYL. Visualization: SYL, YJS, SYH. Writing - original draft: SYL. Writing - review & editing: JYL, YHK.
SUPPLEMENTARY MATERIALS
Supplementary materials can be available at https://doi.org/10.21053/ceo.2018.01914.
An individualized profile in each subject



