Changes in Vestibular Symptoms and Function After Cochlear Implantation: Relevant Factors and Correlations With Residual Hearing
Article information
Abstract
Objectives
The aim of this study was to evaluate vestibular function loss after cochlear implantation (CI) and the relationship between vestibular function and hearing changes.
Methods
Seventy-five patients with CI were enrolled and divided into those with normal preoperative caloric function (group I) and those with a normal preoperative waveform in cervical vestibular evoked myogenic potential (c-VEMP) testing (group II). The relationship between hearing and changes in the vestibular system was analyzed preoperatively and at 3 and 6 months postoperatively.
Results
In group I, unilateral weakness on the implanted side was detected in five (7.7%) and eight (12.3%) patients at 3 and 6 months post-CI, respectively. By 3 months post-CI, the total slow-phase velocity (SPV; warm and cold stimulations) was significantly different between the implanted and non-implanted sides (P=0.011), and the shift in total SPV from pre- to post-CI was significantly correlated with the average hearing threshold at 6 months post-CI. In group II, an abnormal c-VEMP was detected on the implanted side in six patients (16.2%) at 3 months post-CI, and in six patients (16.2%) at 6 months post-CI. Significant changes were noticed in the P1 and N1 amplitude at 3 months postCI (P=0.027 and P=0.019, respectively).
Conclusion
Vestibular function and residual hearing function should be afforded equal and simultaneous consideration in terms of preservation.
INTRODUCTION
It is widely acknowledged that electrode insertion during cochlear implantation (CI) may result in iatrogenic damage to the inner ear. Because residual hearing is often lost during the CI procedure, post-CI preservation of residual hearing has been a major focus of research. The preservation rate ranges between 21.4% and 56.5% [1,2]. Recent studies have proposed a variety of techniques for improving the rate of residual hearing preservation, including “soft surgery” [3,4].
The cochlea and vestibule are subsystems of the labyrinth. Although the surgical procedure for CI does not directly involve the vestibular structure, insertion of electrodes into the scala tympani may affect the vestibular structure in various ways, depending on the anatomical and physiological relationships at play. Several scenarios have been proposed to account for vestibular damage ensuing from CI, including direct trauma from electrode insertion, intraoperative perilymph loss, foreign body reaction or labyrinthitis, endolymphatic hydrops, and electrical vestibular stimulation. The prevalence of subjective dizziness post-CI is estimated to be 9.3% [5] and that of vestibular deficit after CI ranges from 39% to 74% [6-9]. The saccule is the part of the vestibular system that is most commonly damaged during the CI procedure, followed by the utricle and semicircular canals [10]. Anatomically, the ductus reuniens connects the endolymphatic space between the cochlear duct and the saccule. If obstruction of the ductus reuniens or cochlear duct occurs as a result of implantation, the endolymphatic flow will be blocked, and consequently, the saccule will collapse [11].
Several studies have analyzed the factors associated with CI that influence hearing preservation, including insertion depth angle, steroid administration, electrode length, surgical approach, and electrode type. In recent years, the contribution of these factors to the preservation of vestibular function has been the subject of extensive and compelling research, but the follow-up periods in these studies were relatively short and decisive conclusions remain elusive [9,12]. In addition, few reports have examined the relationship between residual hearing and vestibular function after CI, although Nordfalk et al. [13] reported that the loss of caloric response was not associated with the loss of residual hearing. We enrolled subjects with normal vestibular function and analyzed serial changes in their respective vestibular symptoms and function. In addition, we evaluated the correlations of vestibular change with other relevant aspects of the CI technique, as well as residual hearing.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Ajou University School of Medicine (IRB No. AJIRB-MEDMDB-18-161). The requirement for informed consent was waived.
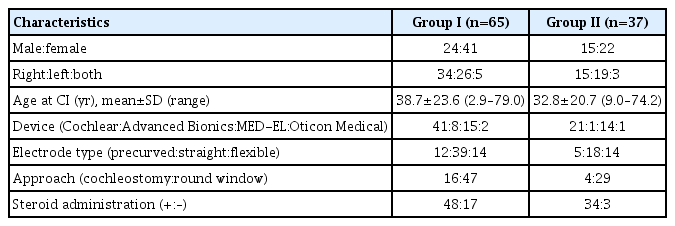
Of 401 patients who underwent CI between February 2002 and December 2017 at the CI Center of the Department of Otolaryngology, Ajou University Hospital, 75 patients for whom the results of hearing and vestibular function tests were available were included in this study. A total of 75 patients were categorized into two conditions, as appropriate, for analyses. Group I (n=65) comprised patients with preoperative normal caloric function; group II (n=37) included patients with a preoperatively normal waveform in cervical vestibular evoked myogenic potential (c-VEMP) testing. Demographic and clinical data, including sex, age, the side on which surgery had been performed, and diagnosis, were retrospectively reviewed (Fig. 1).
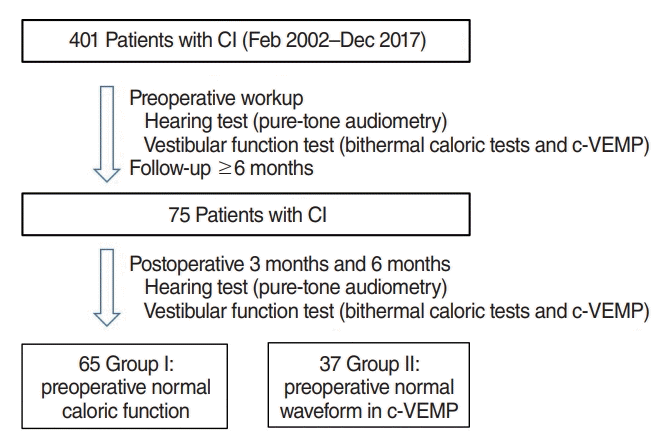
Flowchart of this study. CI, cochlear implantation; c-VEMP, cervical vestibular evoked myogenic potential.
The patient’s subjective dizziness was clinically assessed through face-to-face questionnaire preoperatively and postoperatively. Questionnaire was designed to collect data on the type, duration, and intensity of dizziness. The vestibular function test battery was performed in the following sequence: bithermal caloric tests and c-VEMP tests were performed preoperatively, and again at 3 months, and 6 months postoperatively. Hearing status was also evaluated in accordance with the same schedule, using pure-tone audiometry. The change of residual hearing and vestibular function was compared.
Bithermal caloric tests
Bithermal caloric tests evaluate the degree to which responses in the lateral semicircular canals of each ear are symmetric, by performing two separate stimulations, at 44°C and 30°C, using a water caloric stimulator (NCI-480; ICS). Unilateral weakness (UW) and angular velocity of the slow phase for each stimulation, on both sides, were compared before and after CI. Responses were recorded using a three-dimensional videonystagmography mono-oculography system (SLVNG; SLMED, Seoul, Korea). The caloric test was performed with the patient in a reclining position, with the head turned 30° so that the lateral canal was horizontal. Water was introduced into the ear canal on one side and stopped after 30 seconds, at which point the patient was distracted with quiz questions. Nystagmus usually builds in intensity over around 30 seconds, then gradually decreases over approximately 2 minutes. Each test was performed following a rest of at least 5 minutes; the procedure was repeated for both ears with warm stimulation applied first. Based on the canal paresis values of the bithermal caloric tests for 30 healthy volunteers, we derived 27%, the criteria for the pathologic UW of the equipment in our hospital. A value >27% was deemed to indicate pathological UW.
c-VEMP
The c-VEMP test determines whether the saccule, the otolith, and the inferior vestibular nerve and central connections are intact and working normally. In this study, VEMP analyses were conducted using an evoked potential system (Navigator PRO; Bio-logic Systems Corp., Mundelein, IL, USA). Patients were examined in a supine position, with the head turned in the opposite direction to that of the stimulated ear. The tests were performed using air-conduction tone bursts. Responses were recorded using an active electrode, placed on the mid-point of each sternocleidomastoid muscle, while a reference electrode was placed on the sternum and the ground electrode was placed in the middle of the forehead. The acoustic stimulus comprised a short tone burst, 500 Hz at 90 dB HL, delivered through insert earphones. The duration of the analyses were 100 ms, and the electromyographic signal was band-pass filtered from 500 Hz to 2 kHz. Every set of 200 stimuli was averaged and repeated three times to verify the reproducibility of the response. VEMP asymmetry (VA) was defined as a difference in threshold and/or amplitude between both ears. Abnormal c-VEMP was categorized as the combination of VA or absence of response.
Pure-tone audiometry
Hearing threshold was calculated pre- and postoperatively by averaging low frequencies (250, 500, and 1,000 Hz) and only the patients with functional residual hearing were included in the analysis. Postoperative tests were performed using headphones to analyze the residual audiometric threshold.
Statistical analysis
All data were compared before and after CI, with parametric tests using IBM SPSS ver. 21 (IBM Corp., Armonk, NY, USA). Factors related with subjective dizziness were analyzed using chi-square test. Caloric response and c-VEMP were compared among electrode type, approach, and steroid administration using t-test. The correlation analysis was performed if the change of hearing threshold would be related with the change of caloric response and c-VEMP. In all analyses, a value of P<0.05 was taken to indicate statistical significance.
RESULTS
The study cohort comprised 30 males and 45 females with a mean age of 37.4±23.3 years (range, 2.9–79.0 years) at the time of CI. In total, 43 ears (57.3%) were fitted with cochlear devices (Cochlear Ltd., Sydney, Australia): nine ears (12.0%) were fitted with Advance Bionics devices (Advanced Bionics, Sylmar, CA, USA); 21 ears (28.0%) were fitted with MED-EL devices (MEDEL, Innsbruck, Austria); and two ears (2.7%) were fitted with Oticon Medical Neurelec CI systems (Oticon Medical, Vallauris, France). Regarding the type of electrodes, precurved electrodes (Cochlear Ltd.: 24RCA, 24RECA, and CI512), straight electrodes (Cochlear Ltd.: CI422; Advanced Bionics: HiFocus 1J; Oticon Medical: EVO), and flexible electrodes (MED-EL: Flex 24, 28, and soft) were inserted.
Group I: preoperative normal bithermal caloric function
Among the 75 patients, 65 showed normal bithermal caloric tests preoperatively (Table 1). UW was detected on the implanted side in five patients (7.7%), and in a further eight patients (12.3%) at 3 months and 6 months post-CI, respectively. The maximum slow-phase velocity (SPV) total (from warm and cold stimulation) of the implanted side was 48.4±26.4 preoperatively, 35.3±19.6 at 3 months post-CI, and 37.7±22.6 at 6 months post-CI. The SPV totals of the implanted side and the non-implanted side were compared. While the SPV total (warm and cold stimulations) did not differ significantly between the implanted and non-implanted sides preoperatively (P>0.05), a significant difference was found at 3 months post-CI (P=0.011), but not at 6 months (P=0.133). The shift in the SPV total (i.e., the disparity between the pre-CI and post-CI SPV totals) on the implanted side increased with age at the time of CI at 3 months post-CI (r=0.280, P=0.034), but not at 6 months post-CI.
Of the 19 patients who reported subjective dizziness preoperatively, eight reported the continuation of subjective dizziness at 3 months post-CI, and seven did so at 6 months post-CI. There was no correlation between persistent subjective dizziness postoperatively and UW in the caloric tests at 3 and 6 months postoperatively. Among the patients that did not report any dizziness preoperatively, postoperative dizziness newly developed in eight patients at 3 months post-CI and in five patients at 6 months post-CI. The SPV total was lower among patients with new-onset dizziness (3 months, 26.1±14.9; 6 months, 26.8±16.6) than among the others (3 months, 35.9±19.2; 6 months, 40.3±24.6), but this difference was not significant.
Electrode type, approach, and steroid administration were analyzed in relation to subjective dizziness. The schedule of oral steroid administration was a 10-day course of prednisolone (Solondo; Yuhan, Seoul, Korea) consisting of 60 mg/day for 5 days, 40 mg/day for 2 days, 20 mg/day for 2 days, and 10 mg/day for 1 day. CI was performed on the second day. At 3 months post-CI, subjective dizziness was somewhat more frequent among patients who had undergone cochleostomy (P=0.077), and significantly more frequent among patients who had not been administered steroids (P=0.009). However, no significance was observed at 6 months post-CI (Fig. 2). Postoperative UW was not associated with electrode type, approach, or steroid administration at 3 months and 6 months post-CI. The SPV total was greater in patients whose CI had been performed via the round window approach than in those whose CI was executed via cochleostomy, both at 3 months and 6 months post-CI, but the differences were not significant. The SPV total was also greater in instances of CI with associated steroid administration than in instances of CI without any steroid administration, both at 3 months and 6 months post-CI, but again the difference was not significant. However, the SPV total was greater among patients in whom CI was performed using a flexible electrode (3 months, 43.3±23.0; 6 months, 55.3±26.4) than among those in whom CI was performed using a precurved electrode (3 months, 33.2 ±16.7; 6 months, 27.2±17.4) or straight electrode (3 months, 32.9±18.8; 6 months, 34.5±19.4), both at 3 months and 6 months post-CI, and in this case the difference was statistically significant at 6 months postCI (P=0.007) (Fig. 3).
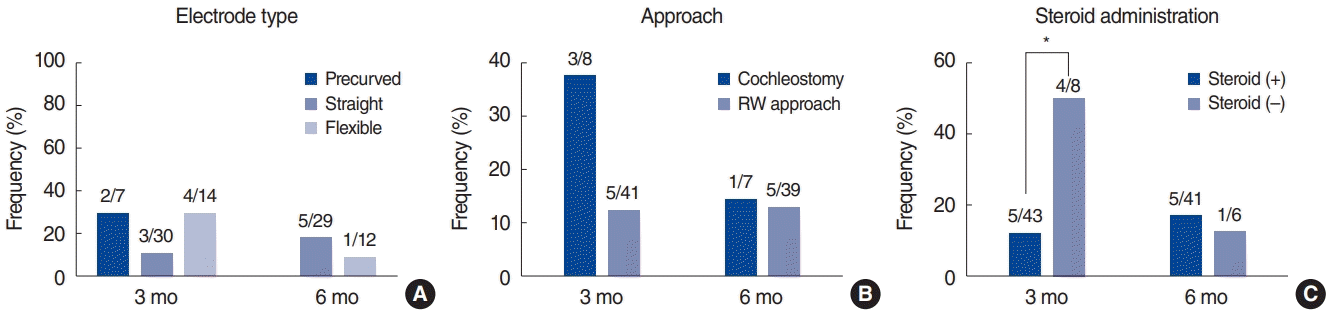
The frequency of new-onset subjective dizziness according to electrode type (A), approach (B), and steroid administration (C). At 3 months post-cochlear implantation, subjective dizziness was somewhat more frequent in patients who underwent cochleostomy (P=0.077), and significantly more frequent in patients who had not been administered steroids (P=0.009). The statistical analysis was performed using the chi-square test. RW, round window. *P<0.05.
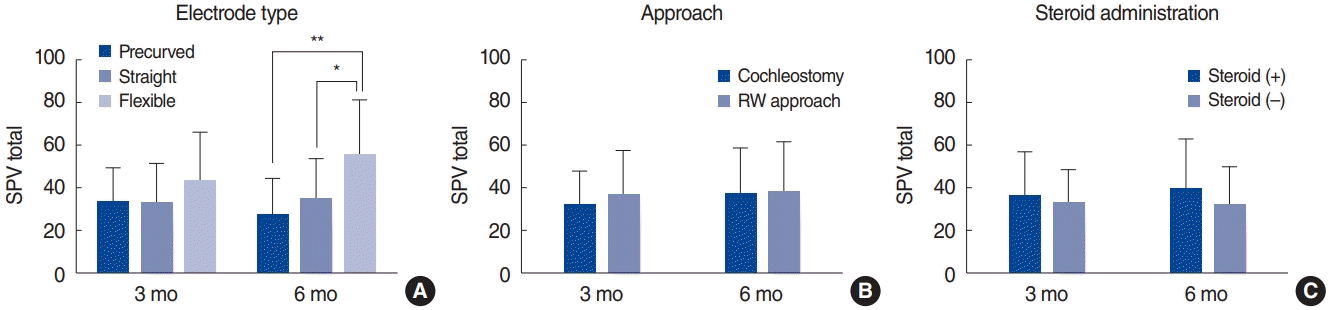
Comparison of the maximum slow-phase velocity (SPV) total (warm and cold stimulations) according to the electrode type (A), approach (B), and steroid administration (C). The SPV total was greater in ears with cochlear implantation (CI) using a flexible electrode (3 months, 43.3±23.0; 6 months, 55.3±26.4) than in ears with CI using a precurved electrode (3 months, 33.2±16.7; 6 months, 27.2±17.4) or a straight electrode (3 months, 32.9±18.8; 6 months, 34.5±19.4), and the difference was statistically significant at 6 months post-CI (P=0.007). The statistical analysis was performed using the t-test. RW, round window. *P<0.05, **P<0.01.
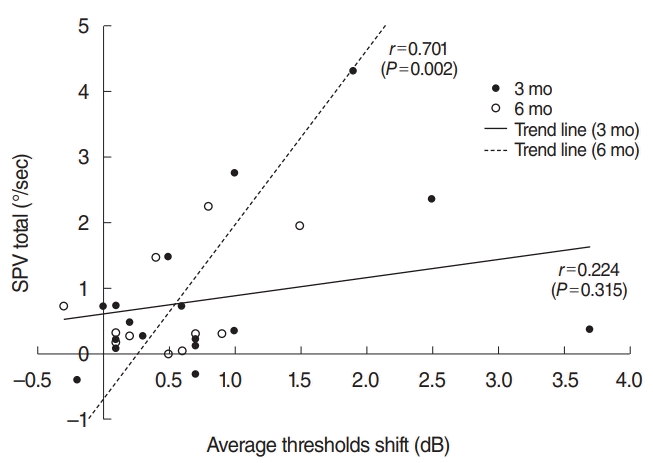
Preoperative and postoperative hearing threshold data were available for 28 patients in group I. The threshold shift (the disparity between the pre-CI and post-CI thresholds) did not significantly differ between patients with UW and those with a normal caloric response at 3 months and 6 months post-CI. However, the shift in the SPV total (corrected for age at the time of CI) was significantly correlated with the average hearing threshold shift at 6 months post-CI (r=0.701, P=0.002) but not at 3 months post-CI (Fig. 4).
Group II: preoperative normal waveform of c-VEMP
Among the 75 patients, 37 patients showed normal c-VEMP data preoperatively (Table 1). An abnormal c-VEMP on the implanted side was detected in six of these patients (16.2%) and in a further six patients (20%) at 3 months and 6 months postCI, respectively. While the P1 latency, N1 latency, and interpeak latency were stationary irrespective of CI, the P1 and N1 amplitude decreased following CI. Parameters were compared between the implanted and non-implanted sides. While all parameters were similar preoperatively (P>0.05), the P1 amplitude and N1 amplitude significantly decreased at 3 months post-CI (P=0.027 and P=0.019, respectively) but not at 6 months postCI. For reference, there was no significant change in the c-VEMP data on the non-implanted side at 3 months and 6 months postCI (P=0.109 and P=0.108, respectively).
Of 13 patients who reported subjective dizziness preoperatively, five reported continued subjective dizziness at 3 months, and three did so at 6 months post-CI. Of the patients who did not suffer dizziness preoperatively, new-onset dizziness was reported postoperatively by five patients at 3 months and two patients at 6 months post-CI. No correlation was detected between subjective dizziness and abnormal changes in vestibular function tests.
Electrode type, approach, and steroid administration were analyzed in relation to subjective dizziness, and no relationship was discerned among them. Postoperative abnormal c-VEMP was also found to be unrelated to electrode type, approach, or steroid administration at either 3 months or 6 months post-CI. Pre- and postoperative hearing threshold data were available for 24 patients in group II. The threshold shift did not differ between the patients with an abnormal c-VEMP and those with a normal waveform at 3 months and 6 months post-CI.
DISCUSSION
Most previous studies have not specifically investigated preoperative vestibular function status. Under such conditions, heterogeneous vestibular data are mixed, meaning that postoperative changes cannot be accurately interpreted. In the present study, patients preoperatively deemed to have normal caloric testing (group I) or c-VEMP results (group II) were analyzed. Generally, canal paresis is calculated using Jongkees’ formula, and in our study, UW was defined as canal paresis in over 27% of cases. Because we intended to analyze changes in canal paresis among the entire cohort of patients, we included the SPV total on the implanted sides as a quantitative factor.
Vestibular function changes were evaluated prior to CI and at 6 months post-CI. The SPV total differed significantly between the implanted and non-implanted sides at 3 months post-CI, while no disparity was observed at 6 months post-CI in group I. Subjective dizziness was somewhat less frequently found depending on the approach, and was significantly less frequently in patients who received steroid administration, but only at 3 months post-CI in group I. In group II, while all parameters were similar preoperatively (P>0.05), the P1 amplitude and N1 amplitude significantly decreased at 3 months post-CI (P=0.027 and P=0.019, respectively). Xu et al. [14] reported that c-VEMP disappearance occurred significantly more often on the implanted side and that waveform parameters showed abnormal changes such as decreased amplitude, forward movement of P1 and N1, and shortened interpeak latency at 1 month after CI, suggesting that c-VEMP waveforms could reflect the degree of damage to the saccule caused by CI. The c-VEMP change in the present study showed a similar tendency at 3 months post-CI. Considering the small sample size, long-term changes in c-VEMP parameters should be explored through follow-up.
Electrode type, approach, and steroid administration may all contribute to postoperative changes in vestibular function. Todt et al. [9] compared VEMP response and electronystagmography and concluded that the round window approach was preferable for preventing vestibular injury. Frodlund et al. [15] reported that precurved electrodes were associated with a significant decrease in SPV and high prevalence of subjective vertigo. Enticott et al. [16]. performed a prospective clinical trial and reported that frequency of postoperative vertigo symptoms were significantly lower in patients who had been administered steroid treatment than the control group. In the present study, the frequency of new-onset subjective dizziness was related to the approach and steroid administration, but UW and an abnormal cVEMP were not related in group I. The SPV total was greater among patients who had been implanted with a flexible electrode than among those in whom precurved and straight electrodes had been used, and this difference was statistically significant at 6 months post-CI in group I.
We also investigated the relationship between residual hearing and changes in vestibular function. The shift in the SPV total was significantly correlated with the average hearing threshold only at 6 months post-CI in group I. This finding indicates that the first 6 months post-implantation may be critical for preserving cochleovestibular system function. It is acknowledged that vestibular function, unlike residual hearing, may be recoverable through central compensation. As such, differences in vestibular function change at 6 months post-CI may be attributable to delayed central compensation. However, asymmetries in caloric function from a stable vestibular lesion could be quite stable long-term, even with central compensation.
Santa Maria et al. [1] reported a linear downward trend in hearing preservation over time. In that study, changes in hearing preservation were particularly significant from 0 to 3 and 0 to 6 months. Vestibular function in our study similarly showed a linear downward tendency over time, and we observed a correlation with residual hearing at 6 months post-CI in group I. We also examined the data from group I to identify characteristics that might predict which patients would suffer UW at 6 months post-CI, but no significant factors were identified (data not shown). A further study based on a large sample is required.
In our study, subjective dizziness was reported by nine patients at 3 months post-CI and six patients at 6 months post-CI in group I. No correlation was found between UW and/or an abnormal c-VEMP and subjective dizziness. There are various causes of subjective dizziness. Rah et al. [17] itemized the etiologies of postoperative dizziness as follows: transient vestibular paresis, benign paroxysmal postural vertigo, endolymphatic hydrops, vestibular migraine, and bilateral hypofunction. However, bithermal caloric tests evaluate the horizontal semicircular canal, while c-VEMP assesses saccular response, and as such, these tests cannot detect subjective dizziness. If the number of samples is sufficient, the SPV total would be expected to be significantly lower in among patients with new-onset dizziness than among others.
Table 2 presents the prevalence of vestibular hypofunction as reported in previous studies. The prevalence rates of UW (12.3%) and abnormal c-VEMP (20%) in this study are lower than the mean rates of UW (31.4%) and abnormal c-VEMP (54.3%) reported in previous studies. This discrepancy might reflect several factors, such as the duration of follow-up, popularization of the soft surgery technique, and perioperative steroid use. Although the extent varies across studies, we may surmise that deterioration of vestibular function is a risk associated with CI. The incidence of abnormal c-VEMP (20%) was higher that of UW (12.3%) in the present study, which is a similar finding to those of previous studies [9,12,18-20]. It is known that the utricle is the sensor that is most frequently damaged after CI [10]. If the ductus reuniens or cochlear duct is obstructed by electrode insertion, endolymph flow could be blocked and the saccule could collapse.
The audiological criteria for CI in the National Health Insurance in Korea are as follows: more than 90 dB of hearing loss at an age ≤24 months (auditory brainstem response threshold), and more than 70 dB of hearing loss at an age >24 months (pure-tone average frequencies of 500, 1,000, 2,000, and 4,000 Hz). Since the residual hearing of high frequencies of most CI candidates is too poor to be considered functional, the average values for low frequencies (125, 250, and 500 Hz) were used for analysis.
There are several limitations of this study that should be considered. Vestibular function data such as the SPV total and cVEMP are known to have high test-retest variability. Further analysis, including a quantitative study, is undergoing. Mastoidectomy and thinning of the posterior canal wall could affect caloric function. Since the caloric test and c-VEMP are primary measurement tools, additional data using the rotational chair test and/or video head impulse test would be needed.
The frequency of post-CI vestibular dysfunction was 12.3% for UW and 20% for an abnormal c-VEMP. The influence of CI on vestibular function was deemed to be potentially critical up to 3 months post-implantation, after which these effects may be minimal. The extent to which residual hearing was preserved was only demonstrably correlated with vestibular dysfunction at 6 months post-CI. Vestibular function and residual hearing should, therefore, be afforded equal and simultaneous consideration in terms of their preservation from the earliest stages of treatment.
HIGHLIGHTS
▪ Caloric testing showed unilateral weakness on the implanted side in five patients (7.7%) and eight patients (12.3%) at 3 and 6 months after cochlear implantation (CI), respectively.
▪ An abnormal cervical vestibular evoked myogenic potential was found on the implanted side in six patients (16.2%) and six patients (16.2%) at 3 and 6 months post-CI, respectively.
▪ Vestibular parameters, such as the total slow-phase velocity and amplitudes of P1 and N1, significantly decreased on the implanted side at 3 months post-CI.
▪ Vestibular function and residual hearing function should be afforded equal and simultaneous consideration in terms of preservation.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: YHC. Data curation: JHJ. Formal analysis: JHJ, HK. Methodology: HYP, OSC. Project administration: YHC. Visualization: OSC. Writing–original draft: JHJ. Writing–review & editing: YHC, OSC.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF2018R1A1A1A05023057)