Precision Medicine Approach to Cochlear Implantation
Article information
Abstract
In the early days of cochlear implantation (CI) surgery, when the types of electrodes were limited and the etiology of sensorineural hearing loss (SNHL) was not well understood, the one-size-fits-all approach to CI held true, as in all other fields. However, in the era of personalized medicine, there have been attempts to associate CI performance with the etiology of SNHL and to establish customized surgical techniques that can maximize performance according to individual cochlear dimensions. Personalized genomic-driven assessments of CI candidates and a better understanding of genotype-phenotype correlations could provide clinically applicable diagnostic and prognostic information about questions such as who, how, and when to implant. Rigorous and strategic imaging assessments also provide better insights into the anatomic etiology of SNHL and cochlear dimensions, leading to individualized surgical techniques to augment CI outcomes. Furthermore, the precision medicine approach to CI is not necessarily limited to preoperative planning, but can be extended to either intraoperative electrode positioning or even the timing of the initial switch-on. In this review, we discuss the implications of personalized diagnoses (both genetic and nongenetic) on the planning and performance of CI in patients with prelingual and postlingual SNHL.
INTRODUCTION
For subjects with severe-to-profound hearing loss who no longer benefit from the use of hearing aids, cochlear implantation (CI) is a better habilitation method for improved speech outcomes [1]. CI is a commonly performed procedure with continuously expanding indications. Speech performance after CI is influenced by a complex array of factors, including the duration of hearing loss, age at implantation, residual hearing, age at onset of hearing loss, type of implant, and socioeconomic status [2,3]. Unfortunately, approximately 3%–7% of cochlear implantees do not benefit from the use of their device [4,5]. Although realistic expectations for CI performance can be predicted to some extent based on prognostic factors, no methods are currently available to identify these potential nonusers prior to surgery.
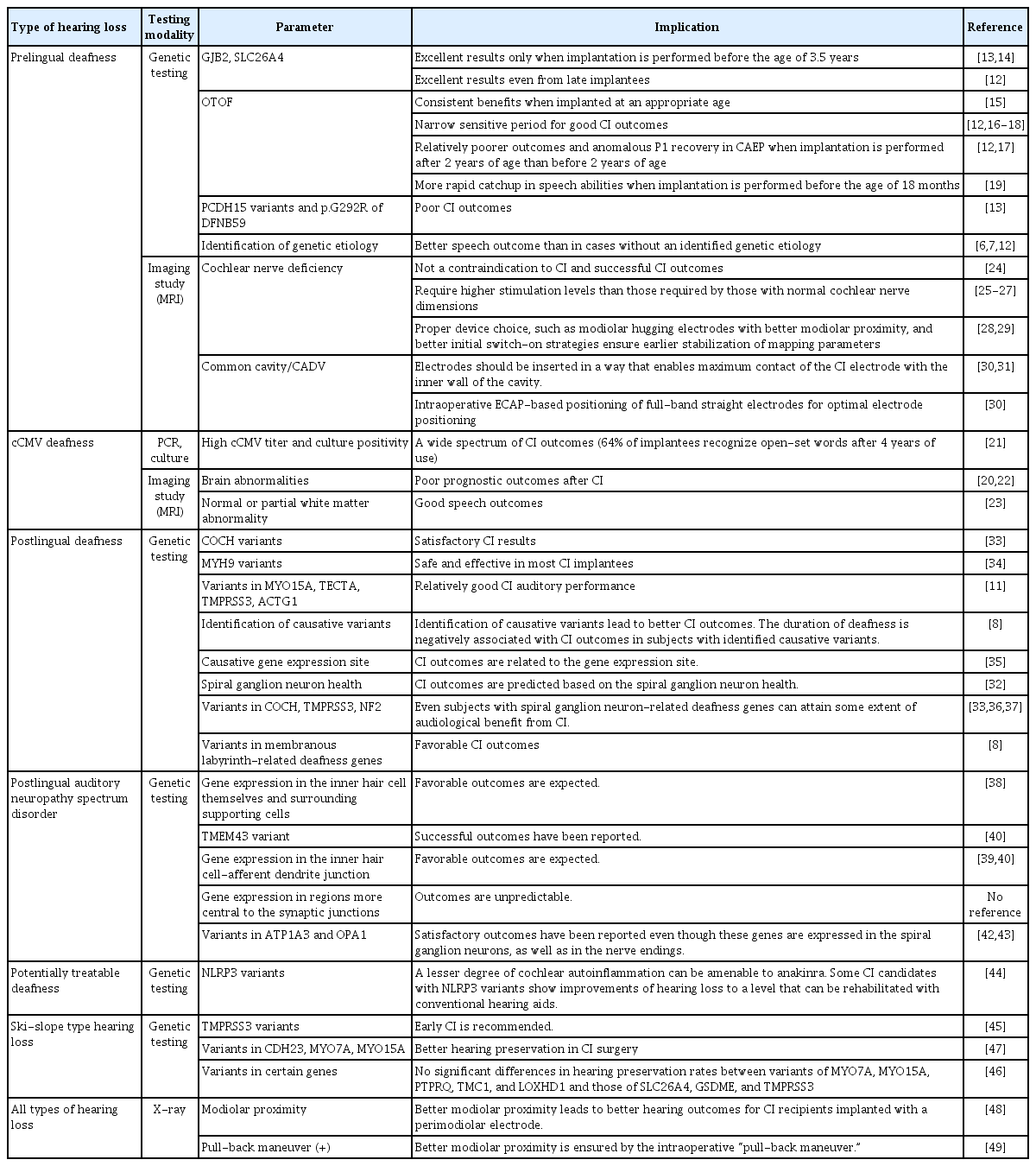
In the era of personalized medicine, attempts have been made to associate CI performance with the etiology of hearing loss [6-8] and to establish surgical techniques that can maximize performance according to individual cochlear dimensions [9,10]. In this review, we discuss the implications of a personalized diagnosis (both genetic and nongenetic) and how it relates to the performance of CI and decision-making in patients with prelingual and postlingual hearing loss (Table 1) [6-8,11-49].
MOLECULAR GENETIC DIAGNOSIS AND CI IN PRELINGUAL DEAFNESS
The introduction of next-generation sequencing technology has allowed implementation of genetic diagnosis in various fields of medicine, including hearing loss [50,51]. Molecular genetic testing (MGT) has now become an important step in the diagnostic workup of CI candidates, providing invaluable information regarding the etiology of hearing loss and the prognosis of CI [11]. This information can predict the natural course of hearing loss, guide patient selection, and aid in determining the timing of CI [52-54].
Hereditary deafness with variants in certain genes is related to especially successful CI outcomes; in particular, CI yields excellent results in patients who have congenital deafness with variants of GJB2 or SLC26A4, the two most common deafness-causing genes [12-14,55,56]. Wu et al. [13,14] found that age at implantation was another important predictor of favorable CI outcomes even in patients with pathogenic variants of GJB2 and SLC26A4, which are genes that allegedly have a good prognosis. Children that received CI before the age of 3.5 years demonstrated better auditory performance at 3 and 5 years post-CI than those without documented pathogenic variants, whereas no significant differences were observed between children that received CI after 3.5 years of age [13,14].
However, in another study of Korean children with GJB2 and SLC26A4 variants who underwent CI, excellent results were also observed in late cochlear implantees [12]. The discrepancy in these findings is attributed to the fact that most late implantees, especially children with SLC26A4 variants, had a history of progressive or fluctuating hearing loss and their pre-CI hearing experience could be associated with their good CI outcomes.
Furthermore, subjects with auditory neuropathy spectrum disorder (ANSD) segregating OTOF variants (DFNB9) showed consistent benefits from CI when implanted at an appropriate age [15]. However, the sensitive period for good CI outcome for DFNB9 individuals may be narrower than that for GJB2- and SLC26A4-related deafness [12,16-18]. Fifty percent of Korean DFNB9 children implanted after 2 years of age showed notably poor outcomes (categories of auditory performance [CAP] scores of 3 and 4) at 24 months post-CI, unlike the CAP scores of 6 and 7 achieved by children implanted early [12]. Furthermore, implantation before the age of 18 months was associated with a more rapid catchup in speech ability after CI [19]. In a recent pilot study investigating the central auditory development of DFNB9 patients after CI, the cortical auditory evoked potential-based P1 components of children implanted after the age of 2 years tended to be “absent” or “anomalous,” almost always associated with delayed language development [17]. Another interesting finding was that when the P1 component was repeatedly measured in DFNB9 patients, even the children who underwent timely implantation (before the age of 12 months) did not achieve sufficient cortical maturation with 6–7 months of device use. This suggests the need for sustained rehabilitation in DFNB9 patients compared to patients with other molecular etiologies.
Bi-allelic PCDH15 pathogenic variants and the p.G292R variant of DFNB59 are reported to be associated with poor CI performance [13]. Pejvakin-deficient mice and humans have been shown to be hypervulnerable to sound because they lack the oxidative stress-induced pejvakin-dependent proliferation of peroxisomes, which contributes to the physiological response to sound exposure [57]. The amplification of sound using hearing aids or CIs may paradoxically worsen hearing impairment in patients with DFNB59 due to sustained injury to the hair cells, spiral ganglion neurons (SGNs), and the auditory nerve by uncontrolled oxidative stress; therefore, the authors suggested antioxidant protection in cases of peroxisomal deficiency, for specific protection against redox homeostasis failure [57].
All in all, implantees with an identified genetic etiology tend to achieve better speech outcomes than those with an unidentified etiology [6,7,12], although some variants are related to poor CI outcomes [13]. The information acquired from MGT can also be used to counsel patients and their families on expected outcomes after CI and the time required to reach those results. MGT can also identify appropriate candidates for personalized and customized auditory rehabilitation among deaf patients.
CI IN CONGENITAL CYTOMEGALOVIRUS INFECTION
Congenital cytomegalovirus (cCMV) infection is a common congenital infection, found in 0.5% to 2% of all live births [58,59]. Some children with cCMV infection can manifest permanent disabilities, including sensorineural hearing loss (SNHL), vision loss, and neurodevelopmental delay, with SNHL being the most common manifestation [60]. Based on the presence of clinical manifestations at birth, cCMV infection can be classified as symptomatic or asymptomatic. Approximately 10% of neonates with cCMV infection are symptomatic (i.e., they are born with clinically apparent sequelae), while the remaining 90% are asymptomatic at birth [61]. However, about 6%–23% of neonates with asymptomatic cCMV infections can also develop late-onset SNHL, while 33%–63% of symptomatic patients develop SNHL [60,62,63].
Resultantly, cCMV infections account for approximately 40% of cases of nongenetically caused congenital SNHL, representing about 20% of all congenital SNHL [64]. A substantial proportion of SNHL cases due to cCMV infections manifest as asymmetrical and progressive hearing loss with significant residual hearing [65]. Indeed, in a study of audiological characteristics in a Korean cohort with cCMV infections, 33.3% of patients had SNHL, 38% had asymmetric hearing loss, 29% had late-onset hearing loss, and there was a diverse spectrum of SNHL severity, ranging from mild to profound [66,67].
Children with significantly delayed speech development due to cCMV-related SNHL are also potential candidates for CI. However, predicting the outcome of prelingual bilateral profound hearing loss due to cCMV infection is not straightforward. Some studies have shown that cCMV patients can achieve comparable CI outcomes to those with idiopathic SNHL [68,69] or SNHL caused by GJB2 variants [70,20]. However, other studies have reported variable outcomes of CI [71,72]. Specifically, Lee et al. [21] found that 64% of children with CMV-related hearing loss were able to recognize open-set words after 4 years of device use, suggesting a wide spectrum of outcomes. Similarly, Viccaro et al. [73] showed that after around 10 years of CI usage, the ability to recognize open-set words improved in most patients. This wide spectrum of outcomes could be attributed to neurodevelopmental delay and cognitive impairment, which can also manifest as a result of cCMV infection. In this sense, brain abnormalities seen on magnetic resonance imaging are considered poor prognostic markers of speech performance after CI [20,22], although they have been shown to be correlated with CI outcomes to some extent [23]. In detail, patients with normal white matter or partial white matter abnormalities on magnetic resonance imaging showed good speech perception performance after CI, at least comparable to the performance obtained by idiopathic SNHL patients [23].
The decision to perform CI in patients with unilateral and asymmetric hearing loss due to cCMV infection is even more complicated. In these cases, since substantial speech development has already been achieved, the decision to perform implantation should be determined by weighing the potential benefits for speech development (i.e., pronunciation or expressive language) that can be obtained through CI against the limitations of development due to cognitive impairment. Specifically, we need to be cautious about performing CI on the worse-hearing ear in a cCMV child with asymmetric hearing loss who has significant cognitive impairment.
CI IN COCHLEAR NERVE DEFICIENCY
Cochlear nerve deficiency, typically defined as a small or absent cochlear nerve in the internal auditory canal (IAC) documented by magnetic resonance imaging, is a known cause of congenital deafness [74,75] and is prevalent in up to 18% of congenital SNHL [76]. The status of the cochlear nerve can be graded based on the number of nerves visualized in the IAC [77]. Grade 0 is defined as no nerves identified in the IAC, grade I as one nerve being present, grade II as two nerves being present, grade III as three nerves being present, grade IV as four nerves being present with a hypoplastic nerve, and grade V as all four normal sized nerves being present in the IAC [77]. However, the limited resolution of magnetic resonance imaging may not accurately reflect the status of the nerves in the IAC [78] and some patients with auditory nerve aplasia do, in fact, respond to electrical stimulation when implanted with a CI [79,80].
The management of hearing loss in children with cochlear nerve deficiency involves many challenges. Because the cochlear nerve is absent or hypoplastic, the electrical signal from the implant provides limited stimulation. Cochlear nerve deficiency can accompany other inner ear malformations, craniofacial anomTable alies, and neurodevelopmental problems, which further influence the outcomes of CI [81,82]. Cochlear nerve deficiency was once considered a contraindication for CI [83], but a growing body of evidence suggests successful CI outcomes in these patients despite imaging evidence of deficient cochlear nerves [24].
Children with cochlear nerve deficiency obviously require higher stimulation levels than those required by those with normal cochlear nerve dimensions [25-27]. These results support the importance of (1) proper device choice (e.g., modiolar hugging electrodes with better modiolar proximity) and (2) appropriate initial switch-on strategies to ensure the earlier stabilization of mapping parameters, thereby maximizing the patients’ performance [28,29].
CI IN COMMON CAVITY OR COCHLEAR APLASIA WITH DILATED VESTIBULE: EXPLORING THE NEURAL TISSUE
CI is generally considered a valid option for common cavity (CC) deformities [84,85], albeit with varying outcomes reported to date. Cochlear aplasia with dilated vestibule (CADV) is traditionally regarded as a contraindication to CI; nonetheless, some satisfactory outcomes have been reported in very small numbers [86,87]. For patients with CC or CADV, the status of the cochlear nerve and the positioning of the electrode can potentially affect CI outcomes [85].
Auditory neural tissues are distributed along the wall of these anomalous cavities [88], which is in line with the clinical observation that a full-band straight electrode outperforms modiolar hugging electrodes in eliciting electrically evoked compound action potential (ECAP) responses in patients with CADV who have undergone CI [30]. Using electrical auditory brainstem response recordings, Yamazaki et al. [89] determined that the auditory neuronal tissue was distributed in the anteroinferior part of CC deformities, mainly near the inner wall of the cavity in all cases. The authors suggested using electrical auditory brainstem response testing to achieve the optimal electrode array placement and to adjust the programming parameters of the implanted device.
During CI surgery for CC/CADV, the electrode should be inserted in a way that enables maximum contact of the CI electrode with the inner wall of the cavity [30,31]. In a previous study, a lower maximum comfortable level and better behavioral outcomes were related to a shorter distance between the inner wall of the CC/CADV cavity and the electrode [90]. Intraoperative ECAP-based positioning of full-band straight electrodes can be implemented in surgical practice to guide the optimal electrode positioning in each individual CC/CADV, allowing successful CI [30].
Taken together, achieving the best possible CI outcomes in CC/CADV depends on the presence of auditory neural tissue and proper positioning of the electrode, which could be assisted by ECAP measurements so that the neural tissues can be fully stimulated.
MOLECULAR GENETIC DIAGNOSIS AND CI IN POSTLINGUAL DEAFNESS
Some genetic variants have been reported to be associated with good auditory performance after CI in postlingual deafness [32, 91,92]. Therefore, identification of pathogenic variants via MGT can be a crucial component in the preoperative evaluation of CI from a prognostic viewpoint. For example, CI is believed to provide satisfactory results in postlingual adult DFNA9 cochlear implantees carrying a variant of COCH that is also expressed in the dendrites of the SGN, in addition to the spiral limbus and the lateral wall [33]. Pecci et al. [34] reported that CI was safe and effective in most patients with MYH9-related disease and deafness. Miyagawa et al. [11] found that four patients with a variant in the MYO15A, TECTA, TMPRSS3, or ACTG1 genes showed relatively good auditory performance after CI including electric acoustic stimulation.
A comprehensive MGT protocol, including exome sequencing, can potentially identify the genetic etiology in approximately 50% of patients with postlingual deafness [8]. Molecular etiologic heterogeneity involving 14 deafness-related genes in 21 subjects was noted in this Korean cohort [8]. Whereas variants of two genes, GJB2 and SLC26A4, account for a high proportion (up to 38%) of prelingual SNHL [12], there is extreme genetic heterogeneity in postlingual deafness [32,91]. Given the nature of this heterogeneity, exome sequencing is often required to identify pathogenic variants.
Implantees whose causative variants were identified among known deafness-related genes yielded better CI outcomes than those without identifiable variants [8]. However, considerable variation in CI outcomes was observed among subjects with the same genotype, meaning that the genetic etiology alone may not be sufficient for predicting CI outcomes. The duration of deafness is negatively associated with CI outcomes, especially in subjects with identified causative variants among known deafness-related genes, but not in those who remain undiagnosed. Therefore, timely CI is recommended in subjects with a known genetic etiology.
CI outcomes are also related to the gene expression site in postlingually deafened cochlear implantees [35]. A classic hypothesis postulated that CI outcomes could be predicted based on SGN health [32]. Membranous labyrinth-related deafness genes, which may inflict relatively weaker damage on the SGN health if mutated, are considered to yield favorable CI outcomes [8]. Furthermore, even subjects with SGN-related deafness genes, including COCH [33], TMPRSS3 [36], and NF2 [37], can attain some extent of audiological benefit from CI.
POSTLINGUAL ANSD AND CI
Perhaps the most substantial beneficiaries of the precision medicine approach to CI are patients with postlingual ANSD. Unlike prelingual ANSD, which is mainly caused by OTOF variants or cochlear nerve deficiency, numerous causative genes of postlingual ANSD have been reported. These genes are broadly divided into genes expressed in (1) inner hair cells themselves, (2) inner hair cell-afferent dendrite junctions, and (3) regions more central to the inner hair cell-afferent dendrite synaptic junctions, depending on their expression sites.
A representative ANSD-related gene expressed only in inner hair cells itself is DIAPH3, which causes lesions limited to the stereocilia of inner hair cells [38]. Many currently known ANSD-related genes are usually expressed at the inner hair cell-afferent dendrite junction. For example, SLC17A8, which encodes VGLUT3, and DMXL2, which encodes rabconnectin-3, are expressed in the synaptic vesicle membrane and are known to cause, if altered, DFNA25 and DFNA71, respectively, in humans [39,40]. In addition to genes expressed in the inner hair cells and the junction itself, some genes are expressed in the supporting cells adjacent to the inner hair cells such as border cells and inner phalangeal cells. The classic example of this is TMEM43 [41]. It is not surprising that favorable CI outcomes are reported among these presynaptic ANSD cases that are not amenable to conventional hearing aids.
Two genes, ATP1A3 and OPA1, merit special attention since these are known to cause syndromic hearing loss. ATP1A3 is the causative gene of CAPOS syndrome (cerebellar ataxia, areflexia, pes cavus, optic atrophy, and SNHL); however, the p.Glu818Lys variant of ATP1A3 was found to lead to a manifestation of ANSD with minimal syndromic features in Koreans. In fact, it frequently appears in the form of nonsyndromic ANSD [42]. Since ATP1A3 and OPA1 are expressed in the spiral ganglia, as well as in the nerve endings of afferent dendrites, the results of CI have been questioned, but satisfactory results have been reported [42,43].
In contrast, when ANSD occurs due to alteration of genes mainly expressed more central to the synaptic region (e.g., the SGN or the cochlear nerve) the outcome of CI is theoretically unpredictable, with residual hearing at risk of aggravation. There are no definitive data on whether these ANSD patients can benefit substantially from CI. Given the lack of a robust clinical test to localize the main lesion of postlingual ANSD, molecular genetic diagnosis is of tremendous importance for predicting the outcomes of CI and sometimes even for deciding whether to perform CI in these patients.
POTENTIALLY TREATABLE SNHL AND CI
The management of hearing loss in a subclass of patients with progressive SNHL due to gain-of-function variants of the NLRP3 gene warrants special attention. The NLRP3 gene encodes the NLRP3 protein, which controls the secretion of interleukin (IL)-1β [93]. The cochlear autoinflammation caused by increased levels of IL-1β in these patients can thus be reversed by systemic administration of an IL-1β antagonist [94]. The degree of hearing loss, and the responsiveness to the IL-1β antagonist (Kineret [anakinra]) may vary from person to person; however, the NLRP3 genotype, auditory thresholds at diagnosis, and radiological findings of the cochlea can collectively serve as potential predictive and prognostic factors of hearing loss progression [95]. Not infrequently, CI candidates with NLRP3 variants show improvement in hearing to a level that can be rehabilitated with conventional hearing aids after daily injections of anakinra, emphasizing the importance of molecular genetic diagnosis in the management of hearing loss. Patients unresponsive to medical therapy nevertheless show excellent audiological outcomes with rapid improvement in speech perception test results, reaching a plateau at 3 months after CI [44].
MOLECULAR GENETIC DIAGNOSIS AND HEARING PRESERVATION IN CI AMONG SKI-SLOPE TYPE HEARING LOSS
A subset of postlingual hearing loss patients exhibits ski-slope type hearing loss—hearing loss with significant low-frequency residual hearing. These patients are in a unique situation because hearing aids do not provide adequate amplification of the mid-to-high frequencies necessary for speech perception; however, many of these patients also do not meet the reimbursement criteria for the insurance system and sometimes do not fall within the conventional candidacy criteria for CI. Therefore, a clinical dilemma exists regarding the decision of when to proceed with CI, and the precision genetic medicine approach can guide decision-making. Specifically, a recent case series describing the effects of CI in children with TMPRSS3 variants has paved the way for the idea of early interventions using electroacoustic stimulation implants in cases where the natural course of hearing loss can be predicted by the genetic etiology [45].
The detection rate of MGT in a Korean cohort of ski-slope hearing loss patients was 37.8% [46]. This number is significantly lower than detection rates of 48%–65% previously reported for SNHL cases diagnosed through the same molecular diagnostic platform [8,53,96]. Considering that around 80% of hearing loss cases are of genetic origin [97], there may be a yet-to-be-found Mendelian genetic disorder behind ski-slope hearing loss. Alternatively, environmental or polygenic factors could play a role in the pathophysiology of ski-slope hearing loss. Nevertheless, the variants found in a ski-slope hearing loss cohort were heterogeneous and included TMC1, TMPRSS3, GSDME, MYO3A, MYO6A, MYO7A, MYO15A, LOXHD1, PTPRQ, SLC26A4, P2RX2, LRTOMT, and USH2A and GPR98 digenic variants [46].
Minimally invasive surgery and delicate electrode array designs have recently allowed hearing preservation in CI surgery, although the hearing preservation rate differs according to studies [98-100]. A trend toward better hearing preservation in genetically diagnosed cochlear implantees has been proposed, especially in patients carrying pathogenic variants of genes specifically expressed in the stereocilia of hair cells [46,47]. Yoshimura et al. [47] found better hearing preservation scores in patients who had pathogenic variants in the CDH23, MYO7A, or MYO15A gene. The authors speculated that the stereocilia function was the key component in residual hearing, and that CI insertion may not affect the residual function of hair cells. However, in the Korean cohort, no significant differences in hearing preservation rates were noted among recipients with genetic variants expressed mainly in the hair cells (MYO7A, MYO15A, PTPRQ, TMC1, and LOXHD1) and those expressed mainly elsewhere in the cochlea (SLC26A4, GSDME, and TMPRSS3) [46]. This issue merits further investigation in larger cohorts.
INFLUENCE OF COCHLEAR PARAMETERS ON CI
Successful CI surgery requires coverage of the optimal frequency range for a good audiological outcome, while avoiding insertion trauma. To achieve a good audiological outcome, closer positioning of the electrodes to the modiolus and robust scala tympani insertion are essential, while the depth of insertion is the most significant factor for the lateral wall arrays [101,102]. Intracochlear positioning of the electrode array nearer to the modiolus leads to better hearing outcomes for CI recipients implanted with a perimodiolar electrode [48], forming the basis for the pull-back maneuver, which has been introduced for slim modiolar electrodes to ensure better modiolar proximity [49].
Cochlear duct length (CDL) has also been considered as another important factor that influences the intracochlear position of the CI electrode and, therefore, CI outcomes [103]. Understanding the CDL has major implications for the electrode array length selection, adjustment of the angular insertion depth, and frequency mapping [104]. However, the CDL and the cochlear size, shape, and spiral characteristics vary even within normal-hearing individuals according to sex and race [105-107]. Based on this, a concept for individualized CI can be presented to optimize audiological outcomes.
A shorter CDL was noted among subjects with congenital deafness than among those with postlingual onset deafness [10]. Short CDL led to a “relative” over-insertion of slim modiolar electrodes and therefore pushed the electrodes further away from the modiolus towards the lateral wall of the cochlea. For subjects with a short CDL, a further pull-back approach—in which the electrode is pulled back by 1 or 2 mm further than in the conventional pull-back approach—was recommended to compensate for the “relative” over-insertion [10].
CONCLUSION
The precision medicine approach to CI refers to a series of processes that determine and customize the preoperative planning of CI, including the decision of whether to perform CI, the timing of surgery, the position of electrodes during surgery, and the timing of the first switch-on of the device, based on the patient’s genome, imaging information, and even the electrophysiological responses obtained from the patient’s cochlea intraoperatively. Recognizing the relevant genotype-phenotype correlations could provide clinically useful diagnostic and prognostic information. Specifically, genetic information may aid in addressing clinical questions regarding who, how, and when to implant and also in identifying individuals with potentially treatable SNHL, thereby avoiding hasty CI surgery. Identifying the nongenetic causes of hearing loss also impacts CI outcomes. Information gathered from a thorough evaluation of imaging studies can direct the timing of surgery, device selection, and insertion techniques to optimize CI outcomes. For certain types of inner ear malformations, the electrophysiological parameters obtained intraoperatively provide clues to the appropriate positioning of the electrodes, and the timing of the initial switch-on of the device has an important effect on the initial rehabilitation process.
HIGHLIGHTS
▪ The precision medicine approach to cochlear implantation (CI) refers to a series of processes that determine and customize the preoperative planning of CI.
▪ Recognizing relevant genotype-phenotype correlations could provide clinically useful diagnostic and prognostic information.
▪ Information gathered from a thorough evaluation of imaging studies can direct the timing of surgery, device selection, and insertion techniques to maximize CI outcomes.
▪ For certain types of inner ear malformations, the electrophysiological parameters obtained intraoperatively provide clues to the appropriate positioning of the electrodes and the timing of the initial switch-on of the device.
Notes
Byung Yoon Choi is an Associate Editor of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: all authors. Funding acquisition: BYC. Methodology: all authors. Project administration: all authors. Writing–original draft: all authors. Writing–review & editing: all authors.
Acknowledgements
This study was supported by Seoul National University Bundang Hospital intramural research fund (SNUBH-13-2022-0010 to Byung Yoon Choi). This study was also supported by the Basic Science Research Program through the NRF, funded by the Ministry of Education (Grant 2021R1A2C2092038 to Byung Yoon Choi).