First Experience of Single-Port Robotic Areolar Approach Thyroidectomy
Article information
Abstract
Objectives
Numerous minimally invasive thyroidectomy techniques have been developed and are actively utilized in hospitals around the globe. Herein, we describe a recently developed minimally invasive thyroidectomy technique that employs the da Vinci SP, and we present the preliminary clinical outcomes of single-port robotic areolar thyroidectomy (SPRA).
Methods
A 3-cm semi-circular incision on the right areola and a small 8-mm incision on the left areola were created. Using hydro-dissection and an advanced bipolar device, a subcutaneous skin flap was created, extending from the areola to the thyroid cartilage. The da Vinci SP was then inserted through the incision in the right areola. Between December 2022 and March 2023, 21 SPRA procedures were conducted. Patients’ medical records and surgical videos were subsequently reviewed.
Results
Lobectomy was performed in 17 patients, isthmectomy in 2 patients, and total thyroidectomy in 2 patients. The mean flap time was 14.9±4.2 minutes and the console time was 62.4±17.1 minutes. The mean tumor size was 0.89± 0.65 cm and the number of retrieved lymph nodes was 3.94±3.98 (range, 0–12). There were no observed instances of vocal cord palsy or hypoparathyroidism.
Conclusion
We successfully developed and performed the novel SPRA for the first time worldwide. Unlike other robotic surgery methods, SPRA is less invasive and leaves no visible scars. This technique employs a sophisticated single-port robotic device. However, to assess the efficacy of this method, we need to analyze more cases and conduct comparative studies in the near future.
INTRODUCTION
Thyroidectomy, a surgical procedure commonly performed worldwide, is used to treat a variety of conditions such as thyroid cancer, suspicious thyroid nodules, hyperthyroidism, and goiter. Among these thyroid disorders, the incidence of thyroid cancers has been on the rise globally over the past few decades, especially in developed countries [1,2]. Consequently, the frequency of thyroid surgery has also increased worldwide, given that the definitive treatment for thyroid cancer involves surgical removal of the thyroid gland and surrounding lymph nodes. As per the national statistics of South Korea, over 30,000 thyroid operations have been conducted annually from 2018 to 2022 [3].
Since the introduction of modern thyroid surgery techniques by Sir Theodor Billroth and Theodor Kocher, the anterior transcervical approach has been in use for over a century and remains the standard procedure for thyroid surgery today [4]. However, this approach inevitably results in a noticeable scar on the front of the neck. Given that thyroid cancer predominantly affects young, socially active women, these visible surgical scars can lead to significant psychological distress [5]. Over the past two decades, there has been a trend towards minimally invasive techniques, including endoscopic and robotic-assisted approaches [6,7]. Among these, the bilateral axillary breast approach (BABA), transaxillary approach (TA), and transoral approach are the most commonly performed worldwide, particularly in robot-assisted surgery [8].
The endoscopic BABA technique was first developed by Professor YK Yoon’s team in 2001, and the same group introduced the robotic BABA method in 2008 [9,10]. The robotic BABA approach not only yields excellent cosmetic results but also ensures oncological safety and low complication rates [8,11]. However, the robotic BABA procedure has inherent drawbacks, as it necessitates the dissection of a broad region of subcutaneous tissue for flap creation, extending from the bilateral axilla and bilateral breast through the anterior chest to the neck [12]. This issue arises from the use of da Vinci surgical robots (S, Si, and Xi), which deploy robotic instruments via four separate robotic arms. In 2018, the single-port-based da Vinci SP system was launched, prompting us to develop a new thyroid surgical technique we’ve named single-port robotic areolar thyroidectomy (SPRA). This method, an evolution of the BABA technique, eliminates the need for bilateral axillary access, thereby minimizing flap dissection. It is less invasive than the BABA method. In this paper, we share our initial experiences with SPRA and provide a detailed description of this innovative surgical technique.
MATERIALS AND METHODS
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Inha University Hospital (No. INH 2023-03-030). The patient photos used in this article were obtained only from patients who had given informed consent after being explained the purpose of medical photography, which is for the evaluation of treatment, research, and education.
Surgical procedure
General anesthesia was administered using endotracheal intubation with NIM-3.0 neuromonitoring tube (Medtronic Xomed) to all patients. The flap design for SPRA is shown in Fig. 1A. A 3-cm circumareolar incision on the right breast was made for da Vinci SP docking. Another 8-mm incision was made for flap dissection and making a channel for suction irrigation. Epinephrine (1:200,000 in saline) was then injected under the subcutaneous layer (hydro-dissection) around the designed flap area, from the breast to the border of the thyroid cartilage. A right 3-cm and left 8-mm circumareolar incision were created and blunt dissection using a vascular tunneller was performed. Further flap dissection was performed using an advanced bipolar device, as previously reported [12]. After flap elevation from the breast and neck, the da Vinci SP trocar was placed through the right areolar incision, as shown in Fig. 1B. An 8-mm trocar was installed on the left side, which was used as a passage for air circulation and small instruments. The da Vinci SP robot was docked with the right 3 cm diameter trocar, as shown in Fig. 1C.
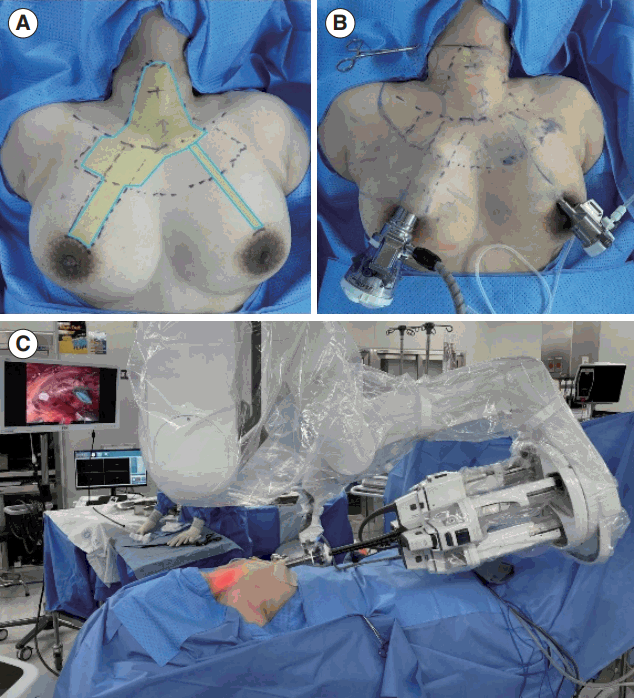
Creation of the flap for single-port robotic areolar thyroidectomy (SPRA) and docking of the da Vinci SP. (A) The process of creating the flap for SPRA. (B) The status of port insertion for SPRA. (C) The docking status of the da Vinci SP for SPRA.
After the robot was docked, thyroidectomy proceeded in the order shown in Fig. 2. The midline of the strap muscle and thyroid isthmus was divided (Fig. 2A). The thyroid gland was retracted to the opposite side and the area between the thyroid gland and strap muscle was dissected (Fig. 2B). The thyroid gland was then pulled up and the recurrent laryngeal nerve was identified (Fig. 2C). The inferior parathyroid gland was saved and the thyroid gland was detached from the trachea (Fig. 2D). The thyroid gland was mobilized to the superior pole and Berry’s ligament was dissected (Fig. 2E). The superior parathyroid gland was identified and saved, and thyroidectomy was performed after superior thyroid vessels were coagulated (Fig. 2F). Level 6 central lymph node dissection between the carotid artery and trachea was performed without affecting the recurrent laryngeal nerve (Fig. 2G). After thyroidectomy, the recurrent laryngeal nerve and the two parathyroid glands were well preserved (Fig. 2H). The excised thyroid and lymph node tissues were placed in a plastic bag and extracted through the right 3-cm trocar. The midline of the strap muscle was closed using a barbed suture (V-Loc, Medtronic). One Jackson-Pratt drain was placed under the flap through the left breast wound. The skin incision was closed using the Vicryl 4-0 knot-bearing suture.
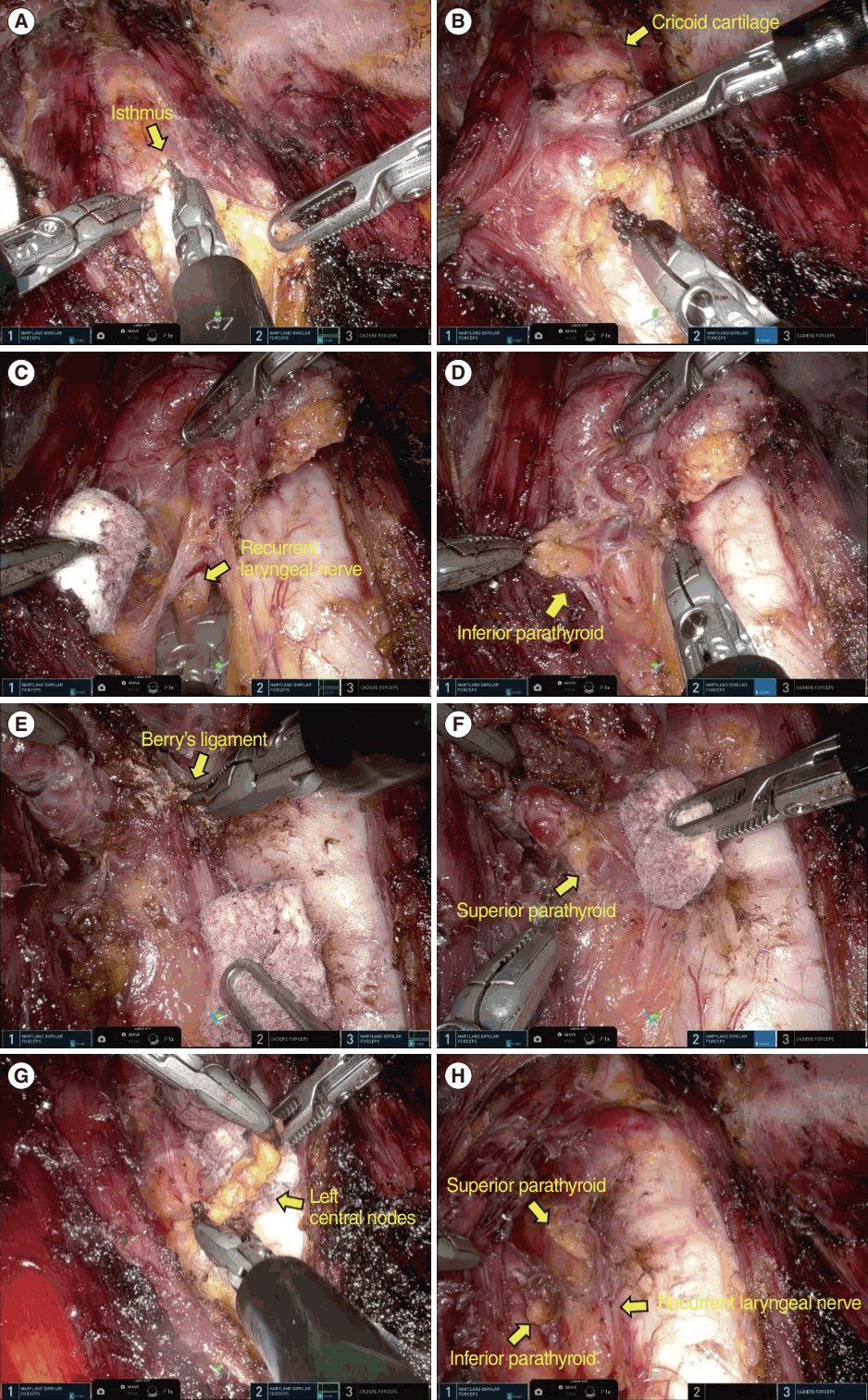
Procedure for the single-port robotic areolar thyroidectomy operation. (A) The strap muscle's midline and the thyroid isthmus were divided. (B) The thyroid gland was moved to the opposite side, and the area between the thyroid gland and strap muscle was dissected. (C) The thyroid gland was then elevated, and the recurrent laryngeal nerve was identified. (D) The inferior parathyroid gland was preserved, and the thyroid gland was detached from the trachea. (E) The thyroid gland was shifted to the superior pole, and Berry’s ligament was dissected. (F) The superior parathyroid gland was identified and preserved, and a thyroidectomy was performed after the superior thyroid vessels were coagulated. (G) A level 6 central lymph node dissection was performed between the carotid artery and trachea, without impacting the recurrent laryngeal nerve. (H) Both the recurrent laryngeal nerve and the two parathyroid glands were successfully preserved.
Both right thyroidectomy and left thyroidectomy can be performed with the same right areolar docking of the da Vinci SP. During the surgery, CO2 gas at 6–7 mmHg pressure and a flow rate of 10–15 L/min was used for inflation. The cable used for neuromonitoring was custom-made by the hospital’s medical engineering department and was selectively connected to the bipolar robotic arm. All surgeries were performed by a single endocrine surgeon in our hospital with an experience in more than 900 robotic BABA thyroidectomy surgeries [13].
Patients
This study included 21 patients who underwent SPRA between December 2022 and March 2023 at our hospital. The surgery was performed by a single endocrine surgeon. Indications for SPRA were as follows: tumor size under 4 cm for benign or follicular neoplasms and under 2 cm for malignant tumors, no radiologic evidence of lateral neck node metastasis, tumor location was not too close to adjacent structures, such as the trachea, esophagus, carotid artery, or jugular veins, no history of surgery or radiation exposure to the neck and breasts, and no severe medical conditions or coagulation disorders. We reviewed the patient’s electronic medical records and surgery videos retrospectively.
Flap time was defined as the time required for flap creation before robot docking, console time was the time that the surgeon spent while sitting on the robotic console to operate the instrument, and total operation time was defined as the total time from the start of the first skin incision to final skin closure. Information on visual analog scales (0 to 10) was collected at 6:00 am were included in statistics for pain score analysis. Patients were discharged if the daily drainage volume was less than 50 mL. For postoperative outcomes, vocal cord function was checked by ENT specialist using laryngoscopy before and after surgery. Hypoparathyroidism was defined as serum parathyroid hormone levels under 10 pg/mL after surgery. Due to the short follow-up period, we only evaluated transient period vocal cord palsy and hypoparathyroidism.
R programming language version 4.2.2 (R Foundation for Statistical Computing) was used for descriptive statistics and for computing the mean and standard deviation.
RESULTS
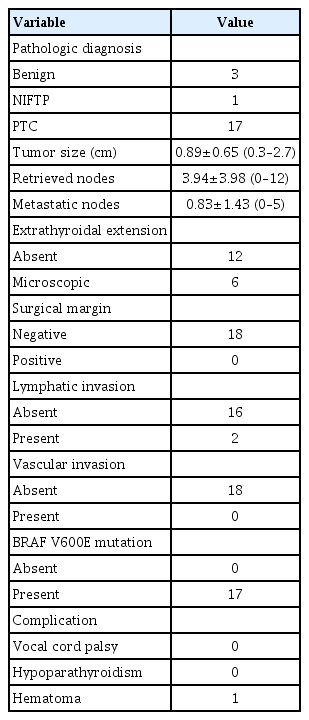
Table 1 displays the clinical characteristics of the 21 patients who underwent SPRA thyroidectomy. The patients, all of whom were women, had a mean age of 41.3±9.9 years, ranging from 29 to 64 years. Their mean body mass index was 23.7±3.56 kg/m2 (range, 18.6–30.0 kg/m2). Fine needle aspiration cytology results revealed malignancy in six patients, suspicion of malignancy in 10, follicular neoplasm in four patients, and atypical cells of undetermined significance in one patient. The primary tumor was located on the right side in 14 patients, while it was found on the left side in three patients. In two patients, the tumor was situated in the isthmus, and bilateral tumors were identified in two patients.
Regarding surgical extent, isthmectomy was performed in 2 cases, lobectomy in 17, and total thyroidectomy in the remaining cases. The mean flap time before the robot docking was 14.9±4.2 minutes (range, 6–29 minutes) and the console time was 62.4±17.1 minutes (range, 30–103 minutes). The total operation time was 121.7±25.0 minutes (range, 85–165 minutes). The estimated mean blood loss was 24.3±27.3 mL (range, 0–100 mL). The postoperative hospital stay was 2.9±0.6 days (range, 2–4 days). The mean pain score after surgery was 2.8±0.5 on postoperative day 1 and 2.4±0.6 on postoperative day 2.
Table 2 shows the pathologic details and complications. The final pathologic diagnoses were papillary thyroid carcinoma in 17 patients, noninvasive follicular thyroid neoplasm with papillary-like nuclear features in one patient, and benign nodules in two patients. The mean tumor size was 0.89±0.65 cm (range, 0.3–2.7 cm). The number of retrieved central lymph nodes was 3.94±3.98 (range, 0–12). The number of metastatic lymph nodes was 0.83±1.43 (range, 0–5). Microscopic extrathyroidal extension was observed in six patients, but no gross extrathyroidal extension was noted. All surgical margins were clear. Two patients showed lymphatic invasion and no patients had microvascular invasion. The BRAFV600E mutation was observed in 17 patients.
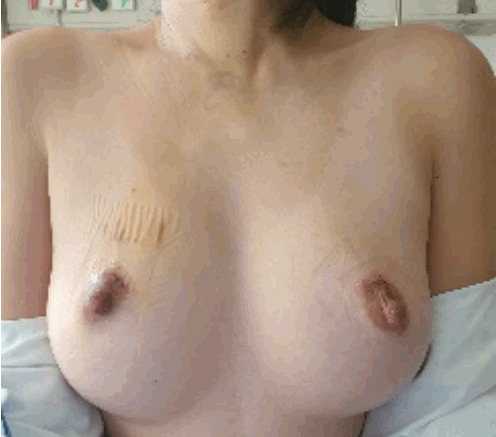
None of the patients exhibited vocal cord paralysis. Similarly, the two patients who underwent total thyroidectomy did not show signs of hypoparathyroidism. One patient experienced hemorrhaging in the area of the right breast where the robot was docked, but this was successfully resolved through hematoma evacuation. The cosmetic results 3 days post-SPRA were excellent, as depicted in Fig. 3.
DISCUSSION
Since the advent of innovative ultrasound imaging and diagnostic techniques in the 2000s, there has been a significant global increase in the incidence of thyroid cancer [1,2,14]. Given that thyroid cancer predominantly affects younger age groups, there has been a growing demand for scarless surgical procedures [15,16]. In response to this, a variety of remote-access thyroid surgical methods have been developed in recent years [7]. Of late, da Vinci robot-assisted thyroid surgery has gained popularity as an alternative to traditional endoscopic or laparoscopic thyroid surgery. The benefits of da Vinci robot-assisted surgery include the ease of controlling surgical instruments, as the robot can mimic the surgeon’s hand movements. Additionally, the technique offers a broad range of motion through its endo-wrist function and superior resolution with full three-dimensional vision, using dual-eye cameras that mimic human eyes [8].
Several representative approaches to robot-assisted thyroid surgery include the TA, BABA, retroauricular, and trans-oral methods [6-8]. The TA robotic thyroidectomy was first introduced by a team led by Professor Woong Youn Chung in 2009, who reported a total of 5000 TA cases by 2018 [17,18]. This TA robotic procedure offers excellent exposure of the thyroid gland from the lateral side and easy access to the lateral neck node area along the jugular vein and carotid artery chain. However, performing surgery on the contralateral thyroid gland can be challenging and requires proper training to ensure appropriate access before the robot docking. This involves making an incision in the axilla, navigating through the sternocleidomastoid muscle, and exposing the thyroid gland. The retroauricular robotic approach, developed by Terris et al. [19], employs a postauricular hairline incision, a technique familiar to head and neck surgeons. Compared to the TA and BABA methods, this approach requires a shorter distance from the incision site to the thyroid gland. However, it has its drawbacks, including a narrow working space and difficulty accessing the opposite side of the thyroid gland. The most recently developed method is the transoral robotic approach, which uses oral vestibular access [20]. This technique requires the shortest distance from the incision site to the thyroid gland and does not necessitate a skin incision. Furthermore, it allows easy access to both thyroid glands and bilateral level 6 neck nodes using a top-down view. However, this technique requires more cumulative experience compared to other methods.
BABA robotic thyroidectomy was started in 2008 at Seoul National University Hospital and more than 5,000 operations have been performed have been successfully performed using this method up until 2023 [10,11,21,22]. This approach offers a comparable operative view of the bilateral thyroid lobes to that of the traditional transcervical thyroidectomy, allowing surgeons to carry out the same operative procedures. With adequate training, surgeons can execute nearly all types of thyroid surgery using the BABA robotic method, including those for large tumors, Graves’ disease, and modified radical neck dissection [23-25]. However, BABA thyroid surgery does necessitate a substantial area for subcutaneous flap dissection and skin incisions in the axilla and breasts to accommodate the docking of four robotic arms. This extensive flap requirement has led some surgeons to view the BABA method as not minimally invasive, but rather maximally invasive [7,8,26]. In an attempt to address this limitation, Kim et al. [27] shared their experience with the “reduced BABA” method, which only utilizes the bilateral axilla and right breast ports. However, this modified method was solely used to perform isthmectomy, and not thyroid lobectomy or total thyroidectomy.
A previous study reviewed BABA thyroid operations conducted since late 2018, detailing the experiences from 500 robotic BABA thyroid procedures [13,28]. In December 2022, our hospital upgraded to the cutting-edge da Vinci SP robotic system. The SPRA method, an evolution of the BABA method, is a minimally invasive technique that utilizes the da Vinci SP. The SPRA method necessitates a smaller flap dissection from the right breast to the neck, thereby eliminating the need for dissection in both axillary areas and reducing the flap area by over 50%, as depicted in Fig. 1A. Once the da Vinci SP is docked, the thyroidectomy procedure can proceed in the same manner as the traditional BABA method (Fig. 2). In the BABA method, the camera port is typically established through the right breast port. Surgeons who have previously performed BABA surgery are familiar with the viewpoint from the right breast port camera. This familiarity is why we first developed SPRA using right breast access. However, SPRA can be adapted to left breast access depending on the patient’s circumstances, such as a history of right breast surgery, or a left-handed surgeon who prefers handling the robotic arm with their left hand.
SPRA thyroid surgery offers several advantages, as it allows for a thyroidectomy to be performed in the same manner as open surgery, utilizing the same field of view. This method also enables operations on both the right and left thyroid glands without needing to adjust the position of the robotic surgical instrument. The time needed for flap creation prior to robot docking is approximately 15 minutes, which is roughly half the time required for the BABA method (30–40 minutes) [12,13]. Moreover, the duration of hospital stay following SPRA surgery is typically shorter than that after BABA surgery (2.86±0.57 days for SPRA vs. 3.48±0.97 days for BABA) [13]. This is attributed to the smaller flap area in SPRA compared to BABA. Consequently, the SPRA method holds an advantage over the existing BABA surgery as it significantly reduces the flap area and is a minimally invasive robotic thyroid surgery. At present, our use of SPRA is limited to tumors smaller than 4 cm, as we are in the initial stages of using this method. As we gain more surgical experience, the surgical indications for SPRA will be expanded. For larger tumors, specimen extraction can be safely accomplished through the right breast port, as the 3-cm diameter of the da Vinci SP trocar site provides about 7.07 cm2 of circular area and the subcutaneous breast tissue is redundant.
Our study has several limitations. Given that this is the first study on SPRA thyroid surgery, the number of cases we examined was relatively small, and we lacked long-term follow-up data. Moreover, we did not conduct a comparative analysis with other surgical methods, such as conventional open surgery or BABA. However, as we accumulate more SPRA cases, we anticipate being able to report on the clinical outcomes of a larger patient group with long-term follow-up, and to conduct a comparative study with SPRA and other surgical methods in the future. Additionally, while total thyroidectomy and central lymph node dissection are feasible with this method, we have not yet attempted lateral cervical lymph node dissection. Similarly, we have not yet performed SPRA on male patients. We expect to overcome these technical limitations as surgeons gain more experience with SPRA. Finally, the surgeon who pioneered SPRA at our institution is highly experienced, having performed over 900 BABA operations, which ensured that the initial results were obtained without issues.
We developed a novel minimally invasive thyroid surgery method, which we named SPRA. This technique only necessitates the docking of the da Vinci SP through a 3-cm access point on the right breast, located along the natural border between the skin and areola. It eliminates the need for subcutaneous flap dissection from the bilateral axillary area, a requirement in the BABA method. Based on our preliminary results, patients have demonstrated positive outcomes without significant complications. However, a larger accumulation of cases will be necessary to facilitate a comparative analysis between SPRA and other robotic thyroid surgery approaches.
HIGHLIGHTS
▪ We developed a new minimally invasive thyroidectomy method using the da Vinci SP, a first of its kind globally.
▪ Single-port robotic areolar thyroidectomy (SPRA) is a minimally invasive procedure that does not result in neck or axillary scars.
▪ The SPRA approach resulted in a reduced flap area compared to the bilateral axillary breast approach method.
Notes
No potential conflicts of interest relevant to this article are reported.
AUTHOR CONTRIBUTIONS
Conceptualization: JWY. Data curation: YSC, JWY. Formal analysis: JWY. Funding acquisition: JWY. Investigation: YSC, JWY. Methodology: JWY. Project administration: JWY, JHC, MSJ, MJY, HML, AYS. Resources: JWY. Supervision: JWY. Writing–original draft: YSC. Writing–review & editing: JWY.
Acknowledgements
This work was supported by Inha University Research Grant.