Differences in Clinical and Immunological Characteristics According to the Various Criteria for Tissue Eosinophilia in Chronic Rhinosinusitis With Nasal Polyps
Article information
Abstract
Objectives
Several criteria exist for classifying chronic rhinosinusitis with nasal polyps (CRSwNP) as eosinophilic or non-eosinophilic. This study attempted to evaluate several criteria for defining eosinophilic CRSwNP from clinical and immunological perspectives.
Methods
A cohort of 84 patients (73 patients with CRSwNP and 11 control patients) was retrospectively analyzed. Patients were divided into eosinophilic and non-eosinophilic CRSwNP based on four different criteria: eosinophils (EOS) accounting for more than 20% of the total inflammatory cells; ≥70 EOS per high-power field (HPF); >55 EOS/HPF; and ≥10 EOS/HPF. Preoperative clinical characteristics, the immunological profiles of 14 cytokines from nasal tissue, and postoperative outcomes were compared between eosinophilic and non-eosinophilic CRSwNP based on each criterion. These criteria were immunologically validated by using 14 cytokines to predict the performance of tissue eosinophilia with a random forest model.
Results
Patients with eosinophilic CRSwNP were significantly older when the criterion of ≥10 EOS/HPF or EOS >20% was used. The number of patients with aspirin intolerance was significantly higher in eosinophilic CRSwNP based on the criterion of EOS >20%. From an immunological perspective, non-type 2 inflammatory cytokines were significantly higher in non-eosinophilic CRSwNP with the criterion of EOS >20% of the total inflammatory cells. In addition, the criterion of EOS >20% of the total inflammatory cells resulted in the best prediction of eosinophilic CRSwNP, with an accuracy of 88.10% and area under the curve of 0.94.
Conclusion
Clinical and immunological characteristics were different between eosinophilic and non-eosinophilic CRSwNP depending on a variety of criteria, and the results of this study should be taken into account when choosing the criterion for defining eosinophilic CRSwNP and interpreting the data accordingly.
INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a common inflammatory upper airway disorder affecting approximately 1%–4% of the population worldwide [1,2]. CRSwNP is a heterogeneous disorder composed of many subtypes, and subtyping based on the inflammatory endotypes—type 2 or non-type 2 inflammation—has been widely accepted, since clinical characteristics and treatment outcomes are often different. For instance, patients with high interleukin (IL)-5 levels have a higher prevalence of comorbid asthma and a higher rate of recurrence [1,3-5]. Biologics targeting type 2 inflammation have been widely used to treat a select population of patients with CRSwNP, especially characterized by type 2 inflammation. The proportions of type 2 and non-type 2 inflammation CRSwNP vary among races, with Asian CRS patients presenting more prominent neutrophilic inflammation than patients from Western countries [6]. In addition, non-type 2 inflammation is known to be more prevalent in younger age groups in Asia, and younger patients with non-type 2 CRSwNP tend to have poorer treatment outcomes than their elderly counterparts [7].
Tissue eosinophilia status is a well-known biomarker indicating whether CRSwNP is type 2 or non-type 2 inflammation. Previous studies have demonstrated the importance of tissue eosinophilia in relation to treatment outcomes after endoscopic sinus surgery [8]. There are several criteria for defining tissue eosinophilia, yet there is no consensus on a definite criterion. Japan and China reported ≥70 eosinophils per high-power field (EOS/HPF) and >55 EOS/HPF, respectively, as cut-offs corresponding to a higher likelihood of recurrence [9,10]. The European position paper on rhinosinusitis with nasal polyps (EPOS) 2020 suggests ≥10 EOS/HPF as the cut-off point when defining evidence of type 2 inflammation for the indication of biological therapy in CRSwNP [11]. Kim et al. [12] employed a novel criterion of EOS accounting for more than 20% of the total inflammatory cells when describing the clinical characteristics and treatment outcomes of eosinophilic CRSwNP in South Korea. Although previous studies have focused on varying treatment outcomes according to the criteria of tissue eosinophilia, no studies have analyzed the clinical and immunological differences between eosinophilic and non-eosinophilic CRSwNP depending on which criteria are used. In this study, the clinical and immunological characteristics and postoperative outcomes were analyzed for eosinophilic and non-eosinophilic CRSwNP based on the aforementioned criteria of tissue eosinophilia.
MATERIALS AND METHODS
Study population
A cohort of 73 patients with bilateral CRSwNP and 11 control patients were recruited from the Department of Otolaryngology Head and Neck Surgery of Seoul National University Bundang Hospital from July 2019 to July 2020. The diagnosis of CRSwNP was based on the criteria described by the European position paper on rhinosinusitis with nasal polyps [11]. All recruited patients with CRSwNP had not responded to medical therapy and underwent functional endoscopic sinus surgery (FESS). Patients who had been treated with preoperative systemic corticosteroids (SCSs) within the 4‐week surgery period were excluded. Nasal polyp tissue samples were harvested during the endoscopic sinus surgery, and for control patients, the ethmoid sinus mucosa samples were harvested during the endoscopic endonasal approach for skull base tumors. All control patients had no evidence of chronic rhinosinusitis at the time of the surgery. Patients who provided written informed consent for participation were analyzed. Patients with unilateral disease, fungal disease, antrochoanal polyps, cystic fibrosis, primary ciliary dyskinesia, or other sinonasal tumorous conditions, such as sinonasal inverted papilloma, were excluded.
This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (No. B-2211-790-301). Written informed consent was obtained from patients.
Patient classification and evaluation of clinical parameters
CRSwNP patients were divided into eosinophilic and non-eosinophilic groups based on the following four criteria: (1) ≥70 EOS/HPF [10]; (2) >55 EOS/HPF [9]; (3) ≥10 EOS/HPF [13]; and (4) EOS accounting for approximately more than 20% of the total inflammatory cells [12]. A detailed histological review was performed at 400× magnification under the microscope. The number of EOS in the mucosa was counted at HPF in three densest areas with cellular infiltrate beneath the epithelial surface, and the mean number of EOS count was calculated. Whether the percentage of EOS exceeded 20% of the total inflammatory cells was determined according to the pathologist’s judgment instead of directly counting all the inflammatory cells. All nasal polyps have been divided into eosinophilic and non-eosinophilic groups by the pathologists based on this criterion from 2006 at our institution. The advantage of this method is its intuitiveness and reproducibility. All cases were reviewed by one experienced pathologist (YBH).
The clinical characteristics including age, gender, blood EOS count, Lund-Mackay (LM) computed tomography (CT) score [14], the ratio of CT scores for ethmoid sinus and maxillary sinus (E/M ratio), and comorbid asthma status were compared between eosinophilic and non-eosinophilic groups. The presence of asthma was assessed by the institution’s allergists after a thorough review of previous medical history and lung function tests, such as spirometry, methacholine challenge test, or bronchodilator response test.
Cytokine measurement from tissue homogenates
All tissue samples were snap-frozen in liquid nitrogen and were stored at –80 ºC. Samples were thawed at room temperature and homogenization was done with a mechanical homogenizer. After homogenization, the homogenates were centrifuged for 20 minutes at 4,000 rpm at 4 ºC. The supernatants were then collected and were stored at −80 °C. These samples were thawed at room temperature, and thorough mixing was done with a vortex shaker. Protein concentrations of tissue extracts were determined using the Pierce 660 nm Protein Assay Kits (Thermo Fisher Scientific). Tissue homogenates were assayed using multiple cytokine analysis kits for detecting macrophage inflammatory protein (MIP)-1β/CCL4, interferon (IFN)-γ, IL-1β/IL-1F2, IL-5, IL-6, IL-8/CXCL8, IL-12 (p70), IL-33, tumor necrosis factor (TNF)-α (Luminex Human Magnetic Assay [9-Plex]; LXSAHM-9; R&D Systems), myeloperoxidase (MPO) (Luminex Human Magnetic Assay [LXSAHM-01]; R&D Systems), tumor growth factor (TGF) β1 (TGFBMAG-64K-01), α1 anti-trypsin (A1AT) (HNDG2MAG36K-01; Millipore), human neutrophil elastase (HNE) (HSP3MAG-63K-01; Millipore), and IL-22 (HTH17MAG-14K-01; Millipore). Data were collected using a Luminex 100 reader (Luminex), and data analysis was performed using MasterPlex QT version 2.0 software (MiraiBio). All assays were performed in duplicates according to the manufacturer’s protocol. All protein levels in the tissue homogenates were normalized to total protein concentration level. The sensitivity for each cytokine is as follows : MIP-1β/CCL4 (5.8 pg/mL), IFN-γ (0.4 pg/mL), IL-1β/IL-1F2 (0.8 pg/mL), IL-5 (0.5 pg/mL), IL-6 (1.7 pg/mL), IL-8/CXCL8 (1.8 pg/mL), IL-12 (p70) (20.2 pg/mL), IL-33 (1.8 pg/mL), TNF-α (1.2 pg/mL), MPO (26.2 pg/mL), TGFβ1 (11.4 pg/mL), α1 anti-trypsin (0.085 ng/mL), HNE (6.2 pg/mL), and IL-22 (0.032 pg/mL). The values less than the detection limit were given equally to half of the lower limit [5].
Evaluation of treatment outcomes
After standard FESS, oral antibiotics were routinely prescribed for five-seven days, and intranasal corticosteroid spray was used afterward. Patients were encouraged to perform saline nasal irrigation for 3 months. Short courses of antibiotics or SCSs were prescribed when necessary. For postoperative outcome, modified Lund-Kennedy (MLK) score, response to SCS use, and exacerbation were compared between eosinophilic and non-eosinophilic groups based on each criterion. MLK score was calculated by adding scores of polyps (0–2), edema (0–2), and discharge (0–2) for both sides [15]. MLK scores were recorded at 3, 6, and 12 months after the operation. For analysis, the MLK scores from 6–12 months after the operation were used. The response to SCS use was analyzed only in patients who were prescribed with SCS at least 1 month after the operation. A patient was considered to be responsive to SCS if the MLK score decreased by at least 50% after the SCS use. Last, exacerbation was defined as an increase of at least 1 point of MLK score during postoperative course along with subjective worsening of the patient’s symptoms.
Statistical analysis
A random forest model was constructed using the normalized values of 14 cytokines measured in tissues from CRSwNP or control with the input variables for immunological validation of patient classification based on tissue eosinophilia status defined by aforementioned criteria. A five-fold cross-validation was used, and accuracy, precision, recall, and area under the curve (AUC) were used to assess the classification performance. In order to identify cytokines that are strongly associated with tissue eosinophilia, the filter method (InfoGainAttributeEval) as the attribute evaluator was used for feature selection, in which features with high scores were identified. The machine learning was performed by Weka [16].
For continuous variables, Student t-test was used for comparison between the two groups, and the three groups were compared using the Kruskal-Wallis test and Dunn’s multiple comparison test after confirming the nonparametric distribution using the Shapiro test. Pearson’s chi-square test or Fisher’s exact test was used for categorical variables. Results are presented as mean± standard deviation. Statistical analyses were performed using IBM SPSS ver. 22.0 software (IBM Corp.). Values of P<0.05 were considered statistically significant.
RESULTS
Preoperative clinical characteristics
Among patients with CRSwNP (n=73), the mean age was 47.4±15.0 years and the male-to-female ratio was 47:26. Fifteen patients were revision cases, 13 patients had comorbid asthma, and four patients had aspirin intolerance. The control group comprised 11 individuals, whose clinical characteristics are described in Supplementary Table 1.
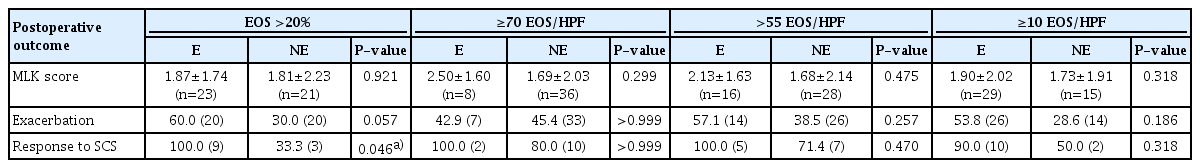
The clinical characteristics of patients with eosinophilic or non-eosinophilic CRSwNP according to different criteria for tissue eosinophilia are presented in Table 1. Blood eosinophilia, age, and the E/M ratio were significantly different between patients with eosinophilic and non-eosinophilic CRSwNP when using criteria with relatively low cut-off values: ≥10 EOS/HPF and EOS >20% of the total inflammatory cells. In addition, aspirin intolerance was associated with eosinophilic CRSwNP when the criterion of EOS >20% of the total inflammatory cells was used. On the contrary, no significant differences were observed in any of the clinical characteristics when the criteria with relatively high cut-off values—≥70 EOS/HPF or >55 EOS/HPF—were used. In addition, the LM CT score, male-to-female ratio, and proportion of patients with comorbid asthma were not significantly different between eosinophilic and non-eosinophilic CRSwNP for all criteria.
Immunological characteristics based on tissue cytokine levels
All mean cytokine levels are listed in Supplementary Table 2, and several important cytokines are described in Fig. 1. The mean IL-5 level was significantly higher in eosinophilic CRSwNP than in non-eosinophilic CRSwNP for all criteria. The mean IL-1β level was significantly lower in eosinophilic CRSwNP than in non-eosinophilic CRSwNP for all criteria except for the criterion of ≥70 EOS/HPF. Interestingly, TNF-α, IL-6, IL-8, and IL-12 levels were significantly lower in eosinophilic CRSwNP than in non-eosinophilic CRSwNP only when the criterion of EOS >20% of the total inflammatory cells was used.

Immunological comparison of eosinophilic and non-eosinophilic polyps based on different criteria. The level of interleukin (IL)-5 (A), a cytokine representative of type 2 inflammation, is significantly different between eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP) regardless of the diagnostic criteria. On the contrary, IL-1β (B), tumor necrosis factor (TNF)-α (C), IL-6 (D), IL-8 (E), and IL-12 (F) were significantly different between eosinophilic and non-eosinophilic CRSwNP only using the criterion of eosinophils (EOS) >20% of total inflammatory cells. E, eosinophilic; NE, non-eosinophilic; HPF, high-power field. *P<0.05, **P<0.01, ***P<0.001.
The classification performance of the 3-class classification (eosinophilic polyp, non-eosinophilic polyp, and control) was the highest when the criterion of EOS >20% of the total inflammatory cells was used to define tissue eosinophilia (Table 2). The accuracy was 88.10%, and the mean AUC was 0.94. Heatmaps of protein concentrations among patient groups according to each criterion are shown in Fig. 2. The segregation between eosinophilic and non-eosinophilic polyps seemed to be the highest with the criterion of EOS >20% of the total inflammatory cells. The filter method for feature selection identified the top five features with high scores: IL-5, IL-8, IL-1β, TGF-β, MPO. Performing cross-validation with the exclusion of each of these features showed changes in accuracy and AUC, with the exclusion of IL-5 resulting in the largest change in both accuracy (88.10–75.00) and AUC (0.94–0.85) (Supplementary Table 3).
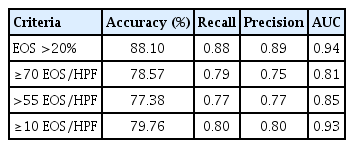
Immunological validation of tissue eosinophilia using a random forest model based on the levels of 14 cytokines from nasal tissues
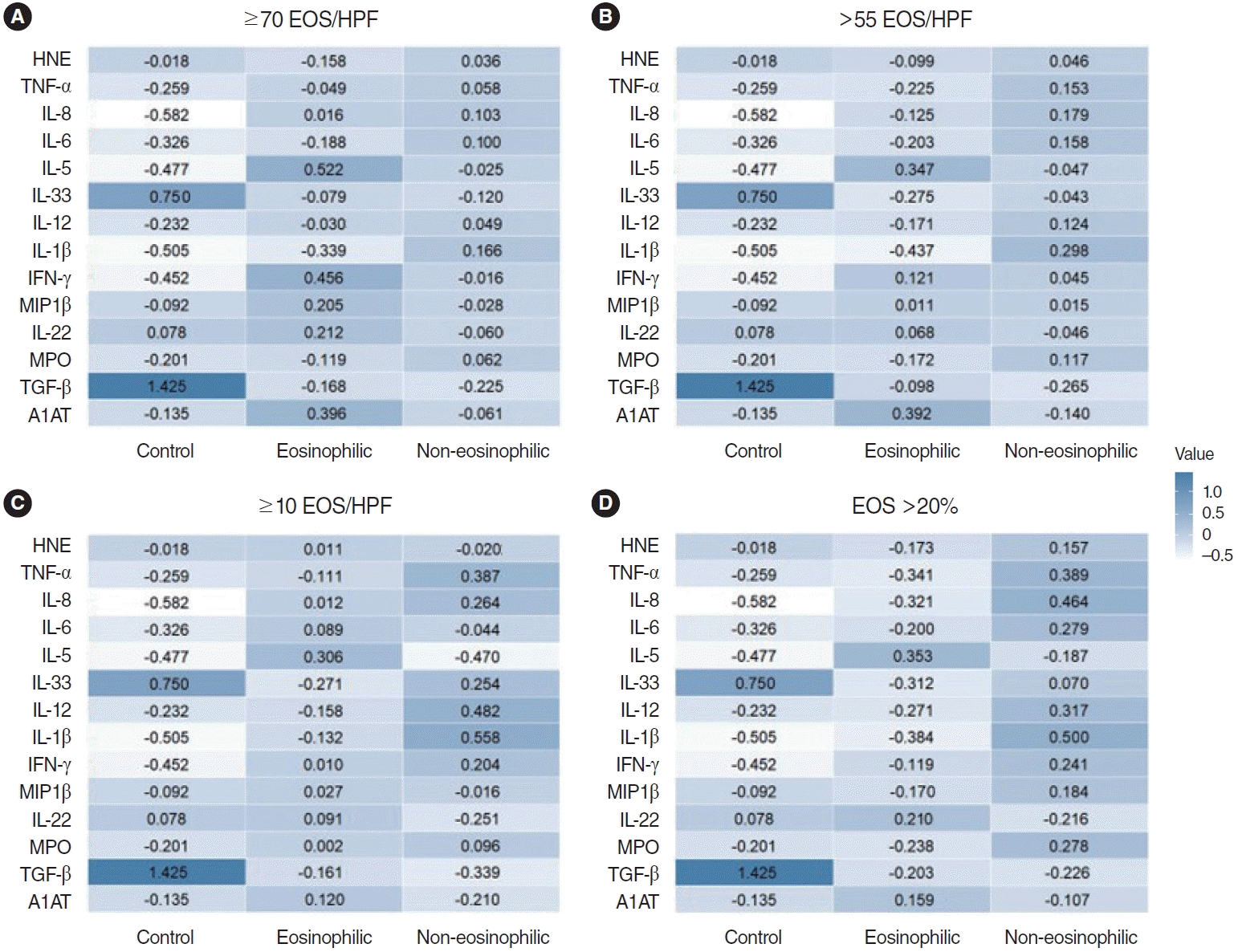
Heatmaps of protein concentrations among the classes based on different criteria for eosinophilia. (A) ≥70 eosinophils (EOS)/highpower field (HPF), (B) >55 EOS/HPF, (C) ≥10 EOS/HPF, (D) EOS >20% of the total inflammatory cells. Color bars present Z-scores of tissue concentration for each measured cytokine. HNE, human neutrophil elastase; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; MPO, myeloperoxidase; TGF, tumor growth factor.
Postoperative outcomes
Postoperative outcomes were analyzed in 44 (60.2%) patients, who visited the outpatient clinic for at least 6 months after the operation. As shown in Table 3, the MLK scores and the exacerbation rates were not significantly different between eosinophilic and non-eosinophilic groups in all criteria. With regard to the response to SCS, a total of 12 patients were prescribed SCS at least a month after the operation, and 10 patients (83.3%) were responsive to SCS. The criterion of EOS accounting for more than 20% of total inflammatory cells was the only criterion in which there was a significant difference in the number of patients responsive to SCS between the eosinophilic and non-eosinophilic groups (Table 3).
DISCUSSION
In this study, we analyzed the differences in clinical parameters and immunological characteristics between eosinophilic and non-eosinophilic CRSwNP based on various diagnostic cut-off values for tissue eosinophilia. Both clinical and immunological parameters were most distinguishable when tissue eosinophilia is defined by the criterion of EOS comprising more than 20% of the total inflammatory cells.
Non-type 2 CRSwNP is known to be predominant at younger ages, and the level of IL-5 is positively correlated with age in CRSwNP patients in Korea [17]. Regarding other clinical parameters, the E/M ratio and the level of blood EOS are both known to be associated with type 2 CRSwNP [18-20]. These findings are in accordance with the current results when the criteria with low cut-off values (e.g., ≥10 EOS/HPF or EOS >20% of the total inflammatory cells) were used. However, these clinical characteristics were not different between eosinophilic and non-eosinophilic CRSwNP when high cut-off values (e.g., ≥70 or >55 EOS/HPF) were used. Patients who are regarded as having eosinophilic CRSwNP using the criteria with low cut-off values may be classified as having non-eosinophilic CRSwNP using the criteria with high cut-off values (Fig. 3A), obscuring the distinction between the two conditions.
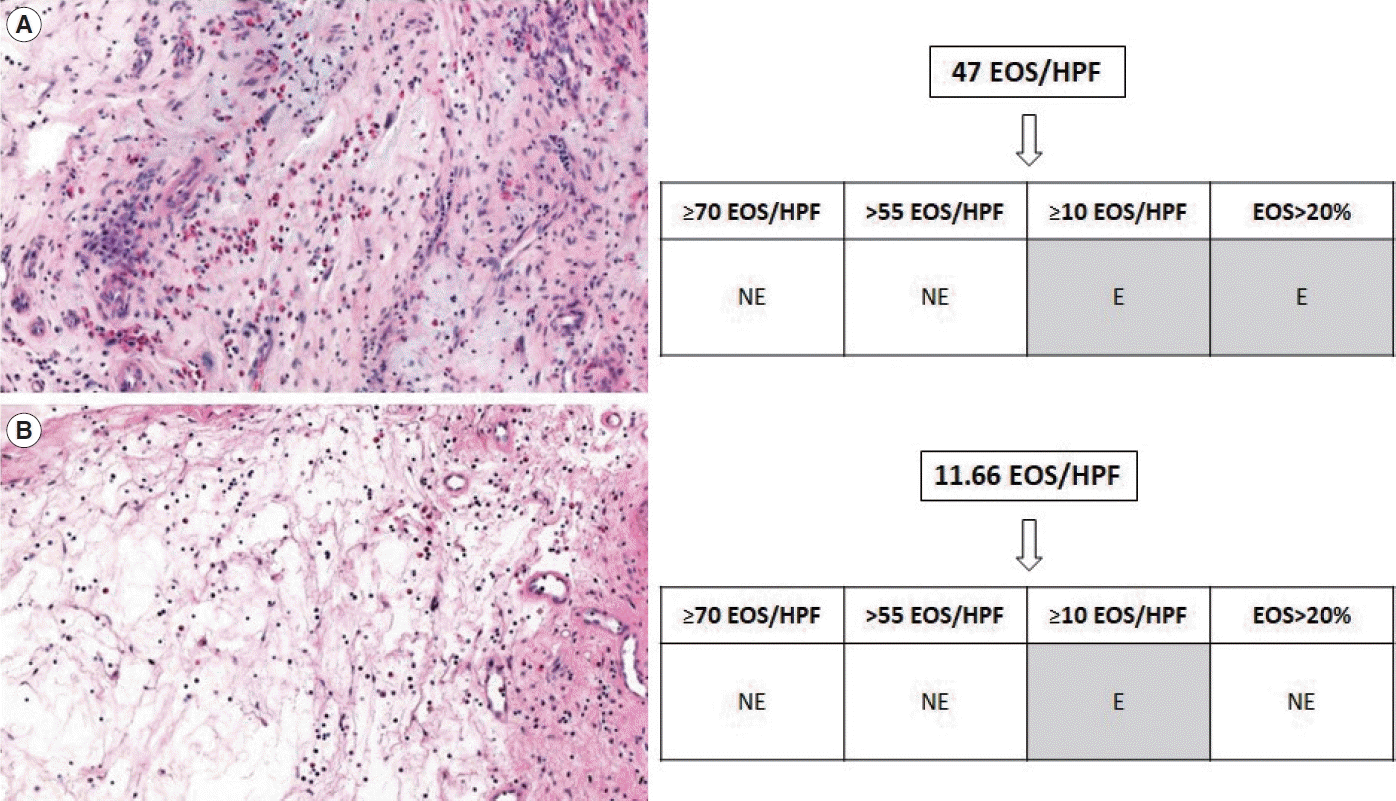
Possible underestimation or overestimation of the expected characteristics of tissue eosinophilia depending on the criteria, as demonstrated by two samples of nasal polyps in hematoxylin and eosin sections (magnification, ×200). (A) 47 Eosinophils (EOS)/high-power field (HPF) may be classified as non-eosinophilic according to the criteria with high cut-off values, while the criterion of EOS >20% of total inflammatory cells classifies it as eosinophilic. (B) 11.66 EOS/HPF may be classified as eosinophilic according to the criterion of ≥10 EOS/HPF, while the criterion of EOS >20% of total inflammatory cells classifies it as non-eosinophilic. NE, non-eosinophilic; E, eosinophilic.
Aspirin intolerance is a risk factor for disease recurrence and is associated with disease refractoriness [21]. Consequently, one might anticipate a higher proportion of aspirin-exacerbated respiratory disease (AERD) among patients categorized as eosinophilic CRSwNP with high cut-off values (e.g., ≥70 or >55 EOS/HPF), as these values are linked to disease recurrence [10,22]. However, our study found no significant difference in the proportion of aspirin intolerance between eosinophilic and non-eosinophilic CRSwNP when using the criteria with high cut-off values. This can be explained by previous findings that there is no significant difference in tissue EOS levels between CRSwNP and AERD [23]. Instead, nasal polyp tissue from AERD patients is known to exhibit high levels of EOS cationic protein [20], which accumulates due to the activation and degranulation of EOS, and may not be detected by traditional hematoxylin and eosin staining.
Comorbid asthma is known to be associated with type 2 CRSwNP, as was also demonstrated in our recent publication [17]. However, this association was not observed in the current study. This discrepancy may be attributed to the small sample size of the study and potential under-diagnosis of comorbid asthma. It is possible that patients with asymptomatic asthma did not seek consultation from allergists. From an immunological standpoint, the level of IL-5, a cytokine indicative of type 2 inflammation, showed a significant difference between eosinophilic and non-eosinophilic CRSwNP, irrespective of the diagnostic criteria used. When IL-5 was excluded, identified via the filter method as the feature with the highest score, there was a substantial shift in both accuracy and AUC in the immunological validation of tissue eosinophilia, as defined by the criterion of EOS >20% of total inflammatory cells. This outcome suggests that IL-5 could serve as a potential biomarker for tissue eosinophilia, aligning with previous research findings [5,18].
Contrarily, significant differences were observed between eosinophilic and non-eosinophilic CRSwNP in terms of IL-1β, TNF-α, IL-6, IL-8, and IL-12 when the sole criterion was EOS >20% of total inflammatory cells. When other criteria were considered, fewer cytokines showed differences between eosinophilic and non-eosinophilic CRSwNP.
IL-1β, a pro-inflammatory cytokine that induces the expression of IL-17, is recognized to be elevated in non-eosinophilic CRSwNP [24]. One potential mechanism in the pathogenesis of chronic rhinosinusitis involves the invasion of an external pathogen into the nasal epithelium. This invasion further stimulates the production of IL-6 and IL-8, markers of neutrophil inflammation, as well as TNF-α, a Th1 marker [25]. A cluster analysis study has shown an increase in IL-6, IL-8, and TNF-α across several non-type 2 endotypes in CRSwNP [5,26]. Furthermore, IL-12 is known to boost the production of IFN-γ by CD4+ T cells [27], so the observed elevation of this cytokine in non-eosinophilic CRSwNP is not unexpected.
The mean values of each aforementioned cytokine in eosinophilic or non-eosinophilic CRSwNP appear to vary based on the criteria used for tissue eosinophilia. Non-eosinophilic CRSwNP, as defined by criteria using high cut-off values, may include patients who could be classified as eosinophilic under different criteria, which could potentially lower the average levels of non-type 2 inflammatory cytokines. Furthermore, a recent study has shown high levels of neutrophils in severe eosinophilic CRSwNP, accompanied by increased Charcot-Leyden crystal deposition and EOS extracellular trap cell death [28]. Therefore, when defined by criteria using high cut-off values, some patients with severe eosinophilic CRSwNP may also exhibit high levels of non-type 2 inflammatory cytokines, such as IL-6 and IL-8, in eosinophilic CRSwNP. Consequently, several non-type 2 inflammatory cytokines may not show significant differences between eosinophilic and non-eosinophilic CRSwNP when high cut-off value criteria are applied.
For postoperative outcomes, no criterion was successful in distinguishing between eosinophilic and non-eosinophilic groups, as evidenced by the MLK score. The definition of eosinophilic CRSwNP as a predictor of disease recurrence may be less impactful for the Korean population, given the prevalence of non-type 2 CRSwNP in Korea. A significant portion of non-type 2 CRSwNP may recur and become resistant cases [29]. The consideration of neutrophilic phenotypes has been suggested in relation to disease severity or steroid resistance in Asian CRS [30]. As such, it may be more practical to categorize eosinophilic CRSwNP patients based on their response to biologics targeting type 2 inflammation. If the eosinophilia cut-off value is set too high, the prediction of an individual’s response to biological therapy could be underestimated. Furthermore, the presence of non-type 2 inflammation in severe type 2 CRSwNP, as defined by high cut-off values, could potentially explain the insensitivity to biological therapy against type 2 inflammation [28]. While EPOS 2020 proposed ≥10 EOS/HPF as the minimum cut-off value to confirm the presence of type 2 inflammation [11], our study found that a 20% cut-off value provided the best performance for immunologic differentiation between eosinophilic and non-eosinophilic CRSwNP. This suggests that this criterion is more representative of the tissue cytokine signature than other criteria.
The response to SCSs is acknowledged as a sign that patients may also respond positively to biological therapy. As such, the current study suggests that the criterion of EOS comprising more than 20% of total inflammatory cells is the most effective predictor of an individual’s response to SCS. This may also imply its potential role in predicting an individual’s response to biologics [31]. However, whether the 20% cut-off value is a superior indicator for evaluating response to biologic therapy requires further validation in future studies.
Eosinophilic CRSwNP, as defined by the criterion of ≥10 EOS/ HPF, may encompass patients who would otherwise be classified as non-eosinophilic according to different criteria, thereby elevating the average levels of non-type 2 inflammatory cytokines. For example, non-eosinophilic CRSwNP, determined by EOS comprising less than 20% of the total inflammatory cells, might actually contain more than 10 EOS/HPF. This would categorize them as eosinophilic CRSwNP under the ≥10 EOS/HPF criterion (Fig. 3B). Consequently, the proportion of EOS, rather than the absolute EOS count, as assessed by a seasoned pathologist, appears to yield a more precise classification that accurately reflects anticipated clinical and immunological profiles. For reference, the classification of tissue eosinophilia based on all criteria, and the number of EOS/HPF for each patient, as well as the comparison of EOS/HPF in each criterion, are presented in Supplementary Table 4 and Table 1, respectively.
This study has several limitations. Its design—as a study conducted at a single institution with a small patient sample—may introduce selection bias, and it does not allow external validation. Additionally, variations in genetic or environmental backgrounds, such as those found in Western countries, could lead to different conclusions due to potential shifts in the proportions of type 2 and non-type 2 CRSwNP. The distribution of patients with eosinophilic and non-eosinophilic CRSwNP is dependent on different criteria, which could result in overfitting or underfitting. This study also includes patients who have previously undergone surgery and experienced recurrence. Patients with recurrent disease are known to have a different immunological profile, often with a higher proportion of mixed-type inflammation [32]. Furthermore, the study population includes patients with a history of sinus surgery and/or use of intranasal corticosteroids. The history of sinus surgery could influence the cytokine profile, as the immunological profile is known to differ between patients with primary nasal polyps and refractory nasal polyps—those who have undergone endoscopic sinus surgery two or more times [32]. Intranasal steroid spray has been reported to decrease intra-epithelial EOS [33]. Finally, the pathologists’ judgments in determining the 20% cut-off value for tissue eosinophilia may vary from center to center. Therefore, a prospective, large-scale, multi-center cohort study with external validation is necessary.
In summary, the clinical and immunological distinctions between eosinophilic and non-eosinophilic CRSwNP may vary based on the criteria used. Eosinophilic CRSwNP is most accurately identified, both clinically and immunologically, when the criterion of EOS >20% of the total inflammatory cells is applied. Further research is required to confirm whether this criterion can serve as a biomarker for predicting an individual’s response to biological therapy targeting type 2 inflammation.
HIGHLIGHTS
▪ Although a variety of criteria for tissue eosinophilia exists, there is no consensus.
▪ Eosinophilic nasal polyp may be best identified when the criterion of eosinophils accounting for more than 20% of the total inflammatory cells is used.
▪ Future studies should be done concerning a criterion which can best predict one’s response to biological therapy.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: SWC. Methodology: SWC. Formal analysis: SWC. Data curation: SWC, YBH. Visualization: JWK, TBW, CSR. Project administration: JWK, TBW, CSR. Funding acquisition: SWC. Writing–original draft: SWC, SKY. Writing–review & editing: SWC, SKY, YBH.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2019R1C1C1009886).
Supplementary materials
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2023.00542.
Clinical characteristics of the control group
Comparison of immunological characteristics of eosinophilic polyps, non-eosinophilic polyps, and control based on different criteria
Immunological validation of tissue eosinophilia using a random forest model with the exclusion of each of top five ranked features
Classification of enrolled patients based on different criteria for tissue eosinophilia and the number of tissue EOS