Findings of Intravenous Gadolinium Inner Ear Magnetic Resonance Imaging in Patients With Acute Low-Tone Sensorineural Hearing Loss
Article information
Abstract
Objectives
Acute low-tone sensorineural hearing loss (ALHL) is thought to have a different etiology from that of idiopathic sudden sensorineural hearing loss. We hypothesized that endolymphatic hydrops (EH) in the inner ear organ contributes to ALHL, even in patients without vertigo. This study investigated the presence of EH in ALHL and compared the clinical characteristics of patients with or without EH.
Methods
We retrospectively reviewed 38 patients diagnosed with ALHL without vertigo from January 2017 to March 2022. EH was measured in all patients using inner ear magnetic resonance imaging (MRI). In addition, we selected patients who showed only mid- or high-frequency hearing loss and had available MRI data as a control group and compared the ALHL and control groups.
Results
After treatment, the pure-tone average at low frequencies significantly improved compared to the initial hearing (P<0.001). Hearing recovery was observed in 63.1% of patients; however, the recovery rate did not differ based on the treatment method. During the follow-up period, six patients (15.8%) progressed to Meniere’s disease, and 18 (47.4%) experienced recurrence. In the ALHL group, the cochlear hydrops ratio on the affected side (0.34±0.09) was significantly higher than on the contralateral side (0.29±0.12) (P=0.005), and most patients showed hydrops in the apex area of the cochlea. Compared with the control group (0.25±0.15), the ALHL group showed a significantly higher cochlear hydrops ratio (P=0.043). The correlation analysis showed a tendency for hearing thresholds at low frequencies to increase as the hydrops ratio increased, albeit without statistical significance.
Conclusion
The cochlear hydrops ratio, especially in the apex area on the affected side, was significantly higher in patients with ALHL, suggesting that EH in the cochlea contributes to the pathogenesis of ALHL.
INTRODUCTION
Acute low-tone sensorineural hearing loss (ALHL) refers to the sudden onset of sensorineural hearing loss confined to low frequencies, with preservation of mid- and high-frequency hearing. In the past, ALHL was regarded as a subtype of sudden sensorineural hearing loss (SSNHL). However, since Abe [1] first reported the clinical characteristics of ALHL as distinct from those of SSNHL in 1982, ALHL has been considered an independent disease. ALHL is frequently accompanied by symptoms such as ear fullness, tinnitus, autophony, and hearing difficulty. Most patients recover hearing within a short period of time without treatment [1,2]. However, because an established definition and treatment strategy for ALHL do not exist, some physicians treat ALHL patients similarly to SSNHL patients.
The etiology of ALHL is uncertain. Previous studies have suggested that autoimmune diseases and migraines play a role in the development and progression of ALHL [3-5]. Several other studies have shown that patients with ALHL have an elevated SP/AP ratio on electrocochleography (ECoG), and some progress to Meniere’s disease (MD). Therefore, endolymphatic hydrops (EH), as in MD, has emerged as an important etiology of ALHL [6-9].
The confirmation of actual EH requires a post-mortem histological examination; therefore, this method has limitations in clinical practice when managing living patients. In 2010, Naganawa et al. [10] established a technique for diagnosing EH using intravenous gadolinium-enhanced inner ear magnetic resonance imaging (IV-Gd MRI), making it possible to visualize EH. In 2018, our institute reported the usefulness of IV-Gd MRI for EH confirmation by revealing that the EH ratio on the affected side in definite MD patients was significantly elevated and correlated with the hearing threshold and ECoG results [11]. IV-Gd MRI currently serves as a helpful supplemental diagnostic tool in clinical practice for diagnosing MD.
In this study, we investigated the clinical characteristics of patients with ALHL and evaluated the presence of EH in ALHL patients using IV-Gd MRI. More specifically, we aimed to determine whether the EH ratio on the affected side in ALHL patients was significantly higher than on the side where low-frequency hearing was preserved. Additionally, we attempted to establish whether a correlation exists between the degree of EH and hearing thresholds at low frequencies.
MATERIALS AND METHODS
Patients and study design
We performed a retrospective medical record review of patients diagnosed with unilateral ALHL who underwent IV-Gd MRI from January 2017 to March 2022. All patients underwent pure-tone audiometry (PTA) in the frequency range from 250 Hz to 8 kHz, and the average values at 0.5, 1, 2, and 4 kHz were used as the average hearing threshold. Among the measured frequencies, 250 Hz and 500 Hz were classified as low frequency, 1 kHz and 2 kHz as mid frequency, and 4 kHz and 8 kHz as high frequency. ALHL was defined as sudden hearing loss ≥30 dB in the low-frequency region (250 Hz and/or 500 Hz) with an average hearing threshold for low frequencies ≥15 dB above that for mid frequencies. Patients who experienced whirling-type dizziness at least once, showed conductive hearing loss, or had retro-cochlear lesions such as vestibular schwannoma were excluded from the study. Finally, a total of 38 patients was included. Electronic medical records including basic patient characteristics, associated symptoms, treatment methods, and hearing thresholds before and after treatment were reviewed.
Because an established treatment strategy for ALHL does not exist, the treatment method for all patients was determined according to the physician’s preference in our institute. Treatment methods included oral or intratympanic steroid therapy as in SSNHL, betahistine and/or diuretics as in MD, or both.
This study was approved by the Institutional Review Board at the Samsung Medical Center following the declaration of Helsinki (No. 2023-02-098-001), and written informed consent was obtained from all patients.
Selection of control groups
This study included two control groups of patients who underwent IV-Gd MRI to compare the EH ratio with the ALHL group. The first control group, MD flat group, included patients diagnosed with definite MD and with a flat hearing configuration >30 dB. Flat hearing was defined as a hearing threshold at all frequencies within 10 dB of the average. The second control group, mid-frequency/high-frequency (MF/HF) group, included patients who showed only mid- or high-frequency hearing loss without vertigo and had normal low-frequency hearing. Finally, 32 and 12 patients were included in the MD flat and MF/HF groups, respectively.
Inner ear MR image scan and analysis
Measurement of EH was performed as described by Naganawa et al. [10]. The inner ear MR image scans were typically conducted within a week of symptom onset, but no later than 1 month after the onset, in a 3.0-Tesla unit (MAGNETOM Skyra, Siemens Medical Solution) using a 32-channel array head coil. All patients initially received a single dose (0.2 mL/kg or 0.1 mmol/kg body weight) of gadobutrol (gadolinium-DO3A-butriol, Gadovist 1.0; Schering) intravenously, and images were acquired 4 hours later. Heavily T2-weighted (hT2W) MR cisternography (MRC) image for anatomical total lymph fluid reference, hT2W three-dimensional (3D) fluid-attenuated inversion recovery image with inversion time of 2,250 ms (positive perilymph image, PPI), and hT2W 3D inversion recovery image with inversion time of 2,050 ms (positive endolymphatic image [PEI]) were obtained in all patients. The HYDROPS (hybrid of reversed image of positive endolymph signal and native image of positive perilymph signal) image was obtained by subtracting the PEI from the PPI. Then, using the DICOM viewer (OsiriX MD image software, ver. 7.5.1 64 bit; Pixmeo Sarl), a HYDROPS-Mi2 image was generated by multiplication of HYDROPS and MRC images. In the HYDROPS-Mi2 image, the endolymph showed negative signal intensity.
Image analysis was performed by a single otologist using the histogram function of the DICOM viewer (OsiriX MD image software, ver. 7.5.1 64 bit). First, a representative MR image slice in which the cochlea and vestibule were best visualized was selected, and a region of interest (ROI) was set by drawing along the contours of the cochlea and vestibule. Then, a copy of the ROI from the MRC image was pasted into the HYDROPS-Mi2 image. The EH ratio was calculated by dividing the number of pixels with negative signal intensity within the ROI by the number of pixels in the total ROI.
Assessment of outcomes
Hearing outcomes after treatment were evaluated based on PTA at 6 months. However, due to the characteristic quick recovery of ALHL, some patients experienced rapid hearing recovery and hence, did not return for further follow-up, making it impossible to assess their hearing at the 6-month mark. In these cases, the last available audiometry results were used for the evaluation of hearing recovery. Hearing recovery was classified into three groups as follows: complete recovery (improvement in low-frequency hearing by ≥10 dB, with an average difference from mid-frequency ≤15 dB), partial recovery (improvement in low-frequency hearing by ≥10 dB but the average difference from mid-frequency was >15 dB), and no recovery (less than 10 dB improvement in low-frequency hearing).
Recurrence was defined as the phenomenon where the hearing returned to “complete recovery” level and was maintained for at least 1 month, followed by a subsequent hearing loss that met the criteria for ALHL. During the follow-up period, two or more episodes of vertigo lasting between 20 minutes and 12 hours accompanied by sensorineural hearing loss in the lowto mid-frequency on the affected side were defined as progression to MD.
Statistical analysis
Statistical analysis was performed with IBM SPSS ver. 26.0 (IBM Corp.). Continuous variables were described as means±standard deviations, and categorical variables were described by frequencies and percentages. Paired t-test was used to compare hearing changes before and after treatment, and Student t-test was used to compare EH ratio between affected and control sides. In addition, Fisher’s exact test was used to determine the difference in hearing recovery based on treatment method, and a simple correlation test was used to evaluate the relationship between EH ratio and hearing threshold. A two-tailed P-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
Among the 38 patients diagnosed with ALHL, 16 (42.1%) were male and 22 (57.9%) were female, with an average age of 49.4±12.3 years. The right side was affected in 17 patients (44.7%) and the left side in 21 patients (55.3%). Associated symptoms included tinnitus (n=27, 71.1%), ear fullness (n=26, 68.4%), and headache (n=4, 10.5%). Twenty patients (52.6%) received oral or intratympanic steroid therapy, nine patients (23.7%) were prescribed betahistine and/or diuretics, and the remaining nine patients (23.7%) received a combination of the two treatments. The average follow-up period was 22.1±31.9 months (range, 1.4–132 months). During the follow-up period, six patients (15.8%) progressed to MD after a mean period of 10.2±7.1 months (range, 1.8–20.6 months). In addition, 18 patients (47.4%) experienced recurrence of ALHL at a mean time of 11.2±19.8 months (range, 0.4–87.2 months) (Table 1).
Hearing outcomes
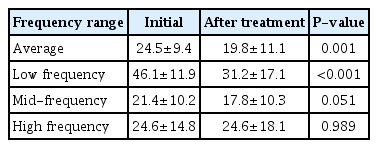
The average initial hearing threshold of all patients was 24.5±9.4 dB. After treatment, the average hearing threshold improved to 19.8±11.1 dB, showing a significant improvement in hearing (P=0.001). Significant changes were not observed in mid-frequency and high-frequency ranges; only low-frequency waves showed significant improvement in hearing, from 46.1±11.9 dB before treatment to 31.2±17.1 dB after treatment (P<0.001) (Table 2). The detailed hearing changes of individual patients are shown in Fig. 1.
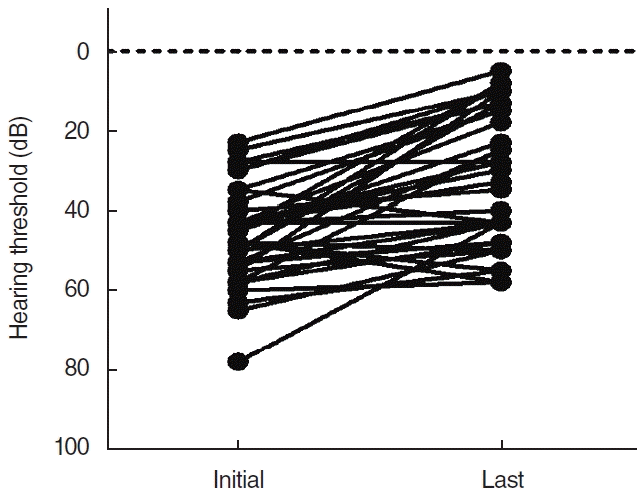
Hearing changes at low frequencies after treatment. The hearing thresholds at frequencies significantly improved from 46.1±11.9 dB before treatment to 31.2±17.1 dB after treatment (P<0.001).
Complete recovery after treatment occurred in 17 patients (44.7%), partial recovery in seven patients (18.4%), and no recovery in 14 patients (36.8%) (Table 1). The complete recovery rate was 40.0% for steroid therapy, 44.4% for betahistine and/or diuretics, and 55.6% for combination treatment. A statistically significant difference was not observed between the recovery rates according to the treatment modality (P=0.444) (Table 3).
EH ratio based on MRI
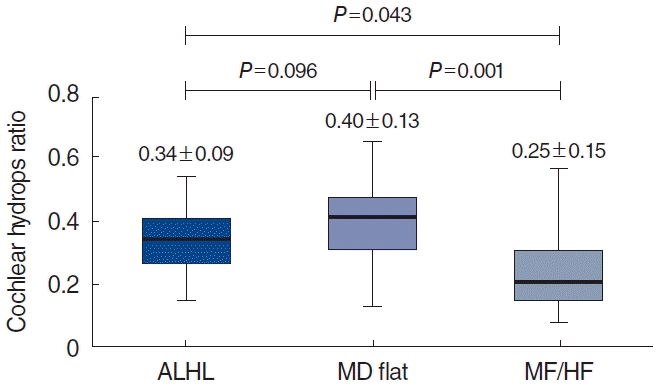
Fig. 2 shows the EH ratio in ALHL patients. Compared with the contralateral side with normal hearing, the cochlear hydrops ratio was 0.34±0.09 on the affected side, which was significantly greater than the value of 0.29±0.12 on the contralateral side (P=0.005) (Fig. 2A). However, the ratio of vestibular hydrops did not show a significant difference between the affected and contralateral sides (0.30±0.17 vs. 0.33±0.17, P=0.205) (Fig. 2B). Because the apex in the cochlea is responsible for low-frequency hearing, the EH ratio was measured separately in the apical turn and mid-basal turn areas of the cochlea (Fig. 3). The cochlear hydrops ratio in the apex region was 0.55±0.21, which was significantly higher than the value of 0.27±0.09 in the mid-base area (P<0.001) (Fig. 2C).
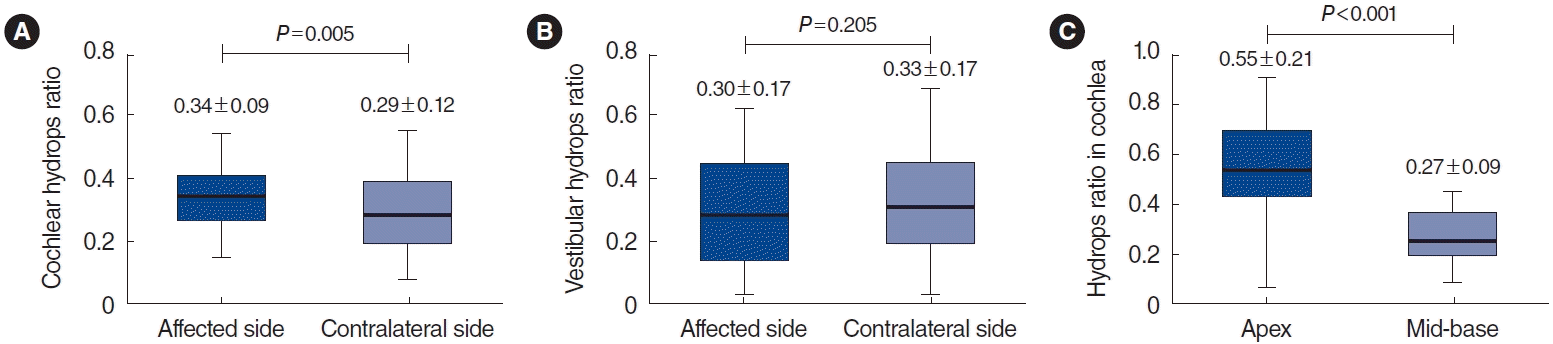
Endolymphatic hydrops (EH) ratio in acute low-tone sensorineural hearing loss patients. Results showing the proportions of (A) cochlear hydrops and (B) vestibular hydrops on the affected side and the contralateral side. (C) Measurements of cochlear hydrops in apical and midbase turns on the affected side. Values are presented as mean±standard deviation.
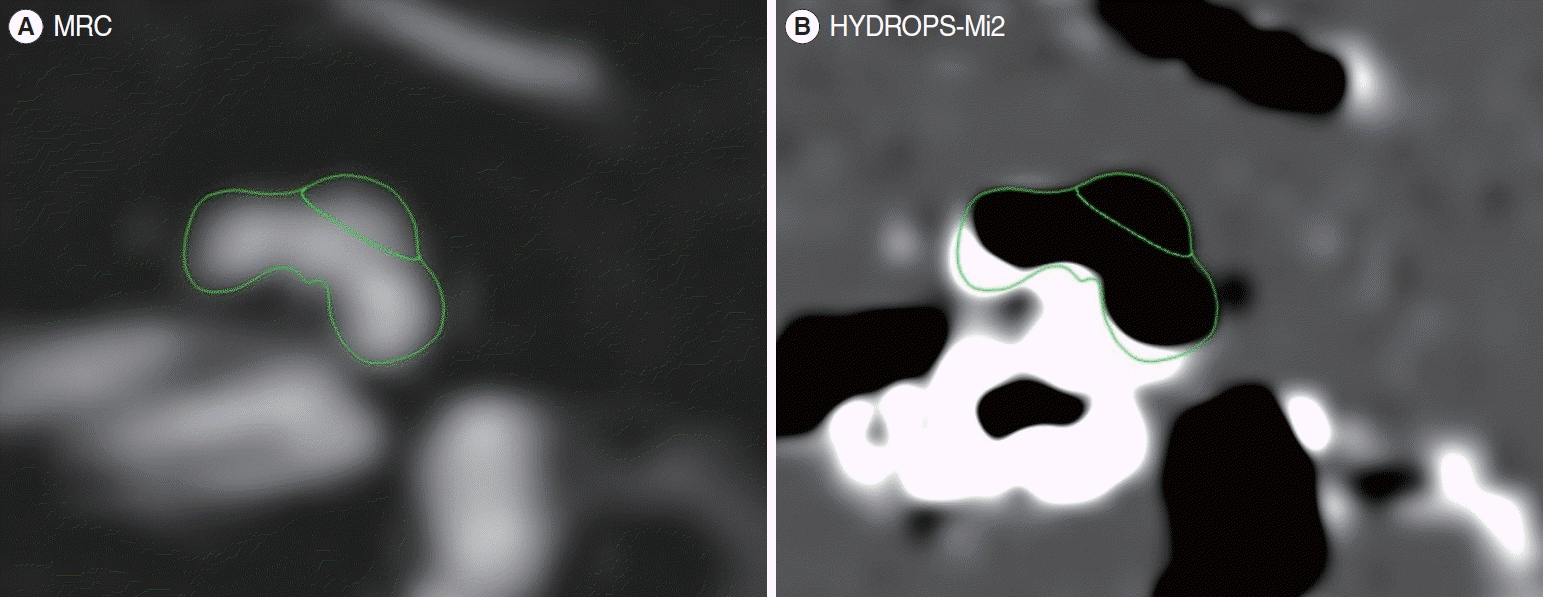
Setting a region of interest (ROI) by dividing the cochlear area. (A) In a magnetic resonance cisternography (MRC) image, the apical turn and mid-base turn of the cochlea were identified, and an ROI was set by drawing along their contours. (B) The previously determined ROI from the MRC image was then copied and pasted into the HYDROPS-Mi2 image. The endolymphatic hydrops ratio was calculated by dividing the number of pixels with negative signal intensity (black area) within the ROI by the total number of pixels in the total ROI.
Thirty-two patients in the MD flat group and 12 patients in the MF/HF group were selected as the control group. In these patients, the cochlear hydrops ratio was also measured in the apical turn and mid-basal turn. Fig. 4 shows the EH ratio of the entire cochlea in each group. A significant difference was observed in cochlear hydrops ratio between the ALHL group and the MF/HF group (0.34±0.09 vs. 0.25±0.15, P=0.043) and between the MD flat group and the MF/HF group (0.40±0.13 vs. 0.25±0.15, P=0.001); however, no significant difference was found between the ALHL group and the MD flat group (P=0.096).
Correlation between EH and hearing threshold
Assuming that EH contributes to the pathogenesis of ALHL, we analyzed whether the severity of hearing loss was associated with the degree of EH. Correlation analysis indicated that the hearing threshold at low frequencies showed a tendency to increase as the cochlear hydrops ratio increased; however, statistical significance was not observed (R2=0.060, P=0.140) (Fig. 5A). Furthermore, a more detailed investigation focusing on the hydrops ratio in the apex region of the cochlea also showed a similar trend, but also without statistical significance (R2=0.029, P=0.308) (Fig. 5B).
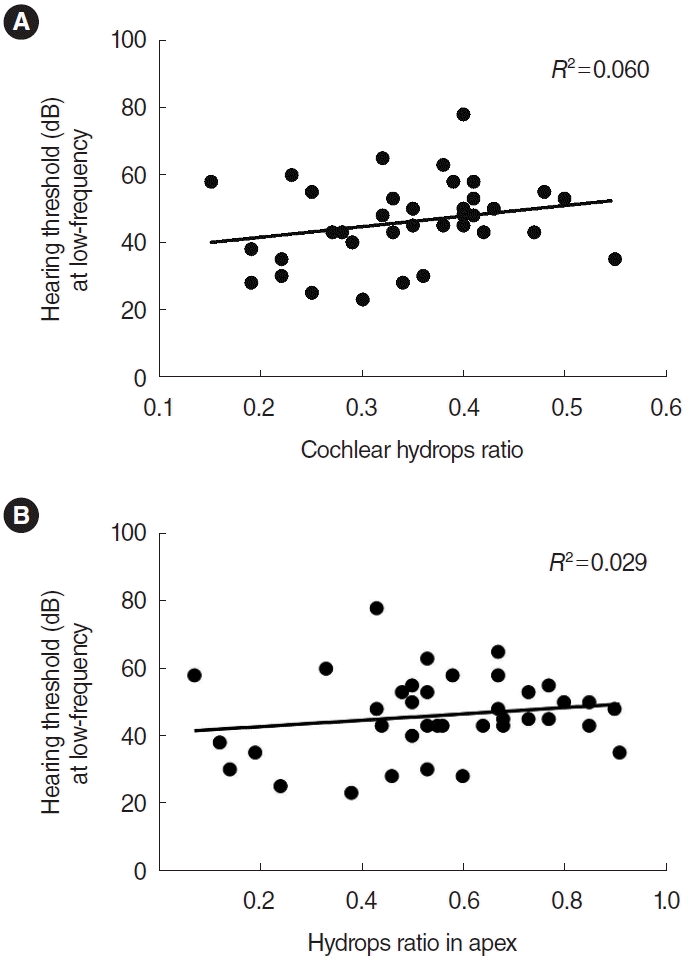
(A) Correlation between the overall cochlear hydrops ratio and hearing threshold at low frequencies. (B) Correlation between the hydrops ratio in the apex and hearing threshold at low frequencies. The correlation analysis showed a tendency for hearing thresholds at low frequencies to increase as the hydrops ratio increased, albeit without statistical significance (P=0.140, P=0.308, respectively).
DISCUSSION
In the present study, we investigated the clinical characteristics of patients with ALHL and the presence of EH in ALHL based on IV-Gd MRI. The results of the study showed a recovery (complete/partial) rate of ALHL of 63.1% and a significant improvement in hearing at low frequencies. No significant difference was observed in hearing recovery based on the treatment method. During the follow-up period, ALHL recurred in 47.4% of patients, and 15.8% progressed to definite MD. The cochlear hydrops ratio on the side with ALHL was significantly higher than on the side with preserved low-frequency hearing.
ALHL shows different characteristics from SSNHL. Imamura et al. [8] reported that ALHL was predominant in females, common in the fourth decade of life, and was accompanied by tinnitus and ear fullness in approximately half of patients. In the study by Yoshida et al. [12] that included patients from a large database in Japan, ALHL occurred at a younger age and was more likely to be bilateral than SSNHL. Similarly, Ma et al. [4] showed that ALHL patients were younger and more frequently female than SSNHL patients. In the present study, tinnitus and ear fullness were observed in more than half of the patients. However, no other prominent features were identified in our study, possibly due to the small number of patients.
The hearing recovery rate in patients with ALHL has been reported in several previous studies to range from 66% to 87%, which is higher than the known recovery rate for SSNHL of approximately 40% [2,6,8,13,14]. The recovery rate of ALHL in our study was 63.1%, which was lower than reported in previous studies but higher than that of SSNHL. In addition, there was no significant difference in the hearing recovery rate based on the treatment method. In a previous study, high-dose steroids were more effective than low-dose steroids for the treatment of ALHL [13]. In another study, combination therapy with steroids and diuretics was more appropriate as initial treatment than monotherapy [15]. However, in a meta-analysis published in 2021, no significant difference was found in the therapeutic effects of steroids and diuretics [16]. Therefore, the optimal treatment method for ALHL remains a matter of debate, and further studies are necessary.
Another characteristic of ALHL is that many patients have recurrent symptoms and some progress to definite MD. The recurrence rate of ALHL has been reported to vary from 10% to 40%, and a higher recurrence rate (47.4%) was observed in the present study [2,4,8,13,17]. This pattern of recurrence also is similar to MD, which is characterized by low-tone hearing loss and fluctuation in aural symptoms. Because progression to MD was observed in 15.8% of patients, a pathophysiological relationship between MD and ALHL may exist.
The etiology of MD is known to be EH. EH of the scala media causes displacement of the basilar membrane toward the scala tympani, damaging spiral ganglion cells and hair cells, which contributes to initiation and progression of symptoms. A possible reason why hearing loss occurs at low frequencies is because the basilar membrane of the apical portion, which is responsible for low-frequency hearing, is wider and softer than that of the basal portion [18]. In addition, the lack of a bony structure supporting the apical lamina spiralis is thought to be another cause [19]. In the present study, the cochlear hydrops ratio was significantly higher on the affected side than on the contralateral side in the ALHL group. The ratio was significantly higher in the ALHL group and MD flat group with low-frequency hearing loss than in the MF/HF group with preserved low-frequency hearing (Fig. 2A, Fig. 4). This supports a previous hypothesis that the apical region is more susceptible to pressure changes caused by EH. Furthermore, the result indicates that EH may have contributed to the pathogenesis of ALHL, as in MD.
Other reports using IV-Gd MRI in MD patients have reported a significant correlation between the degree of cochlear hydrops and the hearing threshold [11]; however, in the present study, statistical significance was not found, although the hearing threshold tended to increase as cochlear hydrops increased. This result may have been due to the small sample size or causes other than cochlear hydrops.
To the best of our knowledge, only a few studies have investigated EH in ALHL using MRI in only a few studies. In 2013, Shimono et al. [20] classified the degree of EH into three categories (none, mild, and severe) based on MRI of 25 patients with ALHL and reported that cochlear hydrops and vestibular hydrops were observed in 92% and 88% of patients, respectively. Cochlear hydrops was significantly more common on the affected side (92%) than on the contralateral side (40%); however, the rate of vestibular hydrops on the affected side (88%) did not differ from that on the contralateral side (85%). These results were consistent with the findings of the present study showing cochlear hydrops to be involved in the pathogenesis of ALHL. However, in the study by Shimono et al. [20], EH was not quantitatively evaluated, which is a limitation of their study; consequently, a relatively high rate of EH (40% of cochlear hydrops and 85% of vestibular hydrops) was reported on the normal contralateral side.
In 2019, Inui et al. [21,22] reported MRI findings in MD and other EH-related diseases, such as SSNHL and ALHL. However, in that study, MD, which is characterized by hearing loss without vertigo and cochlear hydrops on MRI, was classified separately from ALHL. Because not all patients initially diagnosed with ALHL were included, the characteristics of all patients with ALHL were not represented. In the present study, EH was quantitatively evaluated for the first time in patients initially diagnosed with ALHL, which is a strength of the study.
The present study had several limitations. As mentioned above, this study included a small number of patients. In addition, ECoG and other vestibular function tests that can indirectly evaluate EH were not included in the analysis due to the limitations of the retrospective design. Furthermore, our institution does not routinely measure hearing at 125 Hz, a frequency considered in ALHL in many studies. As a result, we had to devise a new diagnostic criterion including only two frequencies for low-frequency hearing loss. This constitutes a unique approach to diagnosing ALHL and may hinder direct comparisons with other studies. Critically, another limitation of our study is that the IV-Gd MRI examinations were not performed prior to the initiation of treatment. The initiation of treatment before the MRI scans potentially influenced the observations of EH, as all MRI examinations were performed during or after treatment. However, even with the potential for treatment to have influenced EH, the significant presence of EH on the affected side supports our research hypothesis that it could be involved in ALHL. Finally, the patients in the present study received different treatments. Even though the hearing recovery results did not differ based on the treatment method, the possibility that use of diuretics affected EH on IV-Gd MRI could not be excluded. Further prospective studies with a larger cohort are necessary.
The present study demonstrated that ALHL shares several characteristics with MD, and the ratio of cochlear hydrops was significantly higher than that of normal low-frequency hearing based on IV-Gd MRI. The results indicate that cochlear EH may contribute to the pathogenesis of ALHL.
HIGHLIGHTS
▪ The cochlear hydrops ratio of the affected side was significantly higher in patients with acute low-tone sensorineural hearing loss compared to the normal side.
▪ The proportion of endolymphatic hydrops in the cochlea was higher in the apex region than in the mid/base region.
▪ As the ratio of endolymphatic hydrops increased, there was a tendency for the hearing thresholds to increase.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: YSC. Data curation: HWS, YK, HJK. Formal analysis: HWS. Funding acquisition: YSC. Methodology: WHC, YSC. Project administration: WHC, YSC. Visualization: HWS, YK, HJK. Writing–original draft: HWS, YSC. Writing–review & editing: WHC, YSC.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (grant number: HR21C0885) and supported by the Electroceutical Technology Development project, National Research Foundation of Korea (NRF), funded by the Ministry of Science (grant no. NRF-2022M3E5E908221711).