Guidelines for the Use of Botulinum Toxin in Otolaryngology From the Korean Society of Laryngology, Phoniatrics and Logopedics Guideline Task Force
Article information
Abstract
The Korean Society of Laryngology, Phoniatrics and Logopedics created a task force to establish clinical practice guidelines for the use of botulinum toxin (BT) in otolaryngology. We selected 10 disease categories: spasmodic dysphonia, essential vocal tremor, vocal fold granuloma, bilateral vocal fold paralysis, Frey’s syndrome, sialocele, sialorrhea, cricopharyngeal dysfunction, chronic sialadenitis, and first bite syndrome. To retrieve all relevant papers, we searched the CORE databases with predefined search strategies, including Medline (PubMed), Embase, the Cochrane Library, and KoreaMed. The committee reported 13 final recommendations with detailed evidence profiles. The guidelines are primarily aimed at all clinicians applying BT to the head and neck area. In addition, the guidelines aim to promote an improved understanding of the safe and effective use of BT by policymakers and counselors, as well as in patients scheduled to receive BT injections.
INTRODUCTION
In the 19th century, botulinum toxin (BT) poisoning, also known as “sausage poisoning,” became recognized in Europe and America. This was due to the consumption of smoked sausages prepared under unsanitary conditions. The term “botulism” was derived from the Latin word for sausage, “botulus” [12]. In 1817, a German physician named Justinus Andreas Christian Kerner managed to extract the toxin from contaminated sausages, and he then outlined the typical symptoms of botulism following a series of experiments on animals and himself. Kerner also described the toxin’s mechanism of action and its potential as a therapeutic agent. Later, during a cluster outbreak of botulism in Belgium in 1893, Émile Pierre-Marie van Ermengem, a bacteriology professor at Ghent University, discovered the bacterium “botulinus” in smoked sausages and post-mortem tissues of patients [13].
In 1949, a study by Burgen revealed the role of BT in blocking neuromuscular transmission by inhibiting the release of acetylcholine from nerve endings. In 1973, Scott explored the potential use of BT in treating strabismus through an experiment involving monkeys. He then conducted the first trial of a botulinum injection on a patient with strabismus in 1977. Following this, BT was identified as a potential treatment for blepharospasm and cervical dystonia in 1985, and it has since been developed for a broad spectrum of therapeutic and cosmetic applications [14]. Specifically, within the field of otolaryngology, BT, as a chemical denervator, is anticipated to be effective in treating various diseases. This is achieved through the reduction of involuntary vocal cord and cricopharyngeal (CP) muscle spasms, enhancement of vocal fold muscle tone, and alleviation of excretory function and pain associated with chronic salivary gland diseases. Although the Food and Drug Administration (FDA) has not yet approved its use for treating diseases within the field of otolaryngology, it is employed as an “off-label” treatment for various conditions. In 1990, both the American Academy of Neurology and the American Academy of Ophthalmology and Otolaryngology endorsed the use of BT as safe and effective for various hyperkinetic diseases.
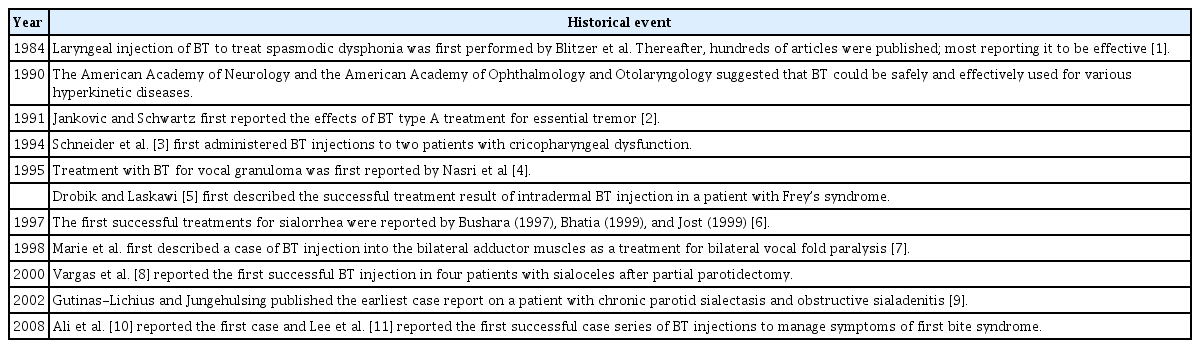
In the field of otolaryngology, BT is frequently employed in the treatment of conditions such as spasmodic dysphonia (SD), essential vocal tremor (EVT), vocal fold granuloma, and salivary gland disease. The historical progression of BT use within otolaryngology is outlined in Table 1. However, a certain level of apprehension surrounding BT persists, leading many physicians to rely primarily on their own experiences when treating patients. Recognizing this, the Korean Society of Laryngology, Phoniatrics and Logopedics (KSLPL) has acknowledged the necessity for a standardized clinical practice guideline (CPG) for the use of BT within otolaryngology.
Types of BT
Clostridium botulinum, a rod-shaped, Gram-positive anaerobic bacterium, has seven serotypes (A, B, C, D, E, F, and G), and each produces a unique neurotoxin [15]. Serotypes A, B, E, and F are toxic to humans, while C and D are only toxic to animals [16,17]. Serotypes A and B are currently commercialized, and serotype A is actively used in clinical practice.
Although BT is marketed as different brands in different countries (Botox [AbbVie Inc.], Xeomin [Merz Pharmaceuticals], Dysport [Ipsen Pharmaceuticals], Neuronox [Medytox Inc.], Botulax [Hugel Pharmaceuticals], and Nabota [Daewoong Pharmaceuticals]), most are manufactured using a similar process. The toxin is manufactured by culturing and fermenting the Hall strain of C. botulinum in an animal-derived medium containing gelatin. Following a purification process, the substance is stabilized with a formulation containing human serum albumin. The toxin is vacuum-dried or freeze-dried (lyophilized) prior to packing in a vial, then shipped in powder form. Sterile saline is mixed into the vial before the toxin is administered to a patient. The dilution and recombination processes each involve a risk of contamination, and using the wrong diluent or an incorrect amount of diluent results in suboptimal clinical efficacy. Although rare, there is a potential risk of unexpected immune reactions or infectious diseases from the animal-derived medium and serum albumin.
Recently, a liquid form of BT that can be used immediately without dilution or recombination (Innotox [Medytox Inc.]) was commercialized. The liquid product uses a medium with no animal-derived substances that contains L-methionine and polysorbate for stabilization and has been approved for safety by the U. S. FDA, instead of the traditional human serum albumin [18]. The toxin is directly transferred into the vial in a liquid state without freezing or vacuum drying. This could reduce the risk of unexpected adverse reactions and inappropriate dilution errors [19]. However, there are still debates about the safety and clinical efficacy of this new formulation, and the market is awaiting additional solid evidence on this issue.
The potency of BT is measured using a mouse unit (MU), where 1 MU is equivalent to the amount of toxin at which 50% of SwissWebster mice weighing 20 g injected intraperitoneally with the toxin die within 3 days. In our study, 1 U corresponds to 1 MU. The lethal dose–50% of BT type A (BT-A) in a 70 kg adult man is 2,500–3,000 U (35–40 U/kg). The toxins most commonly available on the market (type A) have a dose of 50 U or 100 U. Diverse products are released by manufacturers, and the potency of each product varies depending on the product (1 U of Botox [AbbVie Inc.] is approximately as potent as 3 U of Dysport [Ipsen Pharmaceuticals]) [20].
Precautions regarding the administration of botulinum
The most common method of administering BT within the head and neck is a small intra-vocal injection dose of 1–5 U. At this dose, side effects are rare. However, the use of high doses in other areas for neurorehabilitation requires caution. Generally, local reactions at the injection site may occur, such as pain, pulling, swelling, heat, and hypertonia, in addition to systemic side effects, albeit rare. Thus, the recommended dosage and frequency of administration should not be exceeded. According to the approval (precautions for administration) of 50 U of BT-A by the Ministry of Food and Drug Safety in 2018, disorders due to expanded diffusion of the toxin, including muscle weakness, loss of energy, hoarseness, speech disturbance, stuttering, loss of bladder control, dyspnea, dysphagia, diplopia, and ptosis, may occur [21]. Hypersensitivity reactions, such as anaphylaxis, serum sickness, rash, soft-tissue edema, and dyspnea, are also possible.
Patients with preexisting neuromuscular disorders (e.g., amyotrophic axonal sclerosis, motor neuropathy, myasthenia gravis, and Lambert–Eaton syndrome) may be at increased risk of developing significant systemic reactions, even at the usual dose. In most cases, this is caused by weakened respiratory muscles (especially in patients breathing with assisted ventilation) or muscles at the injection site related to the oropharyngeal muscles. Such reactions increase the risk of gastric tube placement and aspiration from severe dysphagia.
Given the previously reported deaths due to severe respiratory failure, it is evident that immediate attention is required for disturbances of swallowing, vocalization, and respiration occurring within days or weeks of treatment. Serious adverse events, including those that lead to fatal outcomes, have been reported in connection to injections of BT in or around anatomically vulnerable structures, such as the salivary glands, mouth, pharynx, esophagus, and stomach. In the United States, 28 deaths were reported between 1989 and 2003 in relation to the non-cosmetic use of BT, mostly due to respiratory failure caused by a diffusion of the toxin [22,23]. The response to BT injections depends on the anatomy, the preparation of the toxin, the dose-response relationship, dissolution, storage following dissolution, and the individual’s immunogenicity.
The remarkable safety of BT injections can be attributed to the ability of the toxin to remain locally at the injection site. Preventing the injected toxin from having an effect beyond the injection site and administering the smallest possible volume with an effective dose is critical for maximizing the dose response and minimizing side effects. That is, a small volume and high dose at the target site is preferable to an injection of a high volume and low dose. As the concentration of BT may differ depending on the preparation, units cannot be interconverted among products. To reduce the risk of potential antibody resistance, the lowest effective dose should be administered and additional injections at intervals of less than two to three months between treatments are discouraged [24].
MATERIALS AND METHODS
Intended users
These guidelines are primarily aimed at all clinicians applying BT to the head and neck area. In addition, the guidelines aim to promote an improved understanding of the safe and effective use of BT by policymakers and counselors, as well as in patients scheduled to receive BT injections.
Organization of the committee and selection of key questions
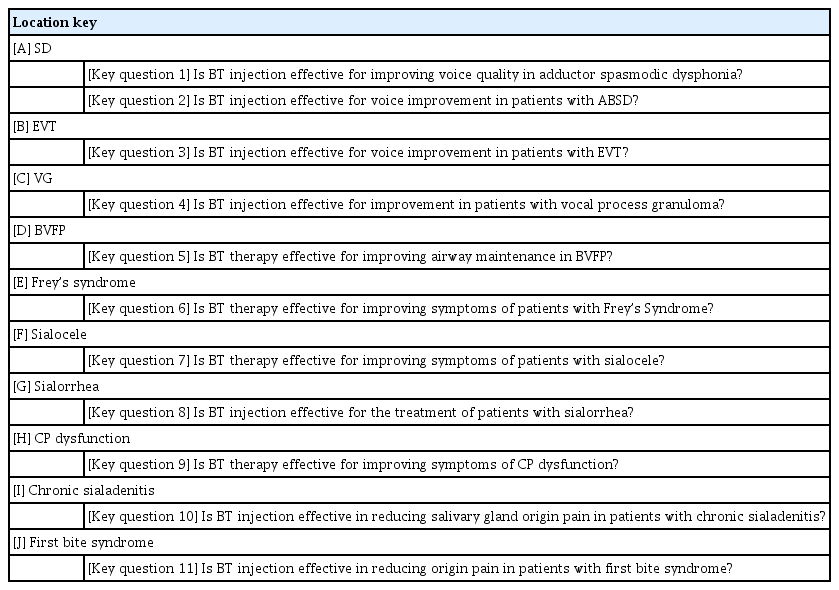
The committee was divided into advisory, managing, and working groups. The managing members included the committee chair (SWL) and two executives (MJB and CHR) appointed by the KSLPL. The advisory and working groups comprised 10 KSLPL members with extensive clinical experience and nine junior faculty KSLPL members in teaching hospitals, respectively. The advisory and managing groups set the subject that required CPG development as “guidelines for the use of botulinum toxin in the otolaryngology field,” and then developed the key questions (KQs) during the first three meetings. They did not include the use of BT injections for aesthetic purposes. A final list of 11 KQs was determined, with topics including SD (KQs 1 and 2), EVT (KQ 3), vocal fold granuloma (KQ 4), bilateral vocal fold paralysis (BVFP) (KQ 5), Frey’s syndrome (KQ 6), sialocele (KQ 7), sialorrhea (KQ 8), CP dysfunction (KQ 9), chronic sialadenitis (KQ 10), and first bite syndrome (KQ 11) (Table 2). The managing and working groups performed the literature search and created the draft. The committee members participated in the development of the CPG independently of the KSLPL. The inaugural meeting of the committee was on April 29, 2021, and a virtual or in-person conference call was held once a month during CPG development.
Literature search
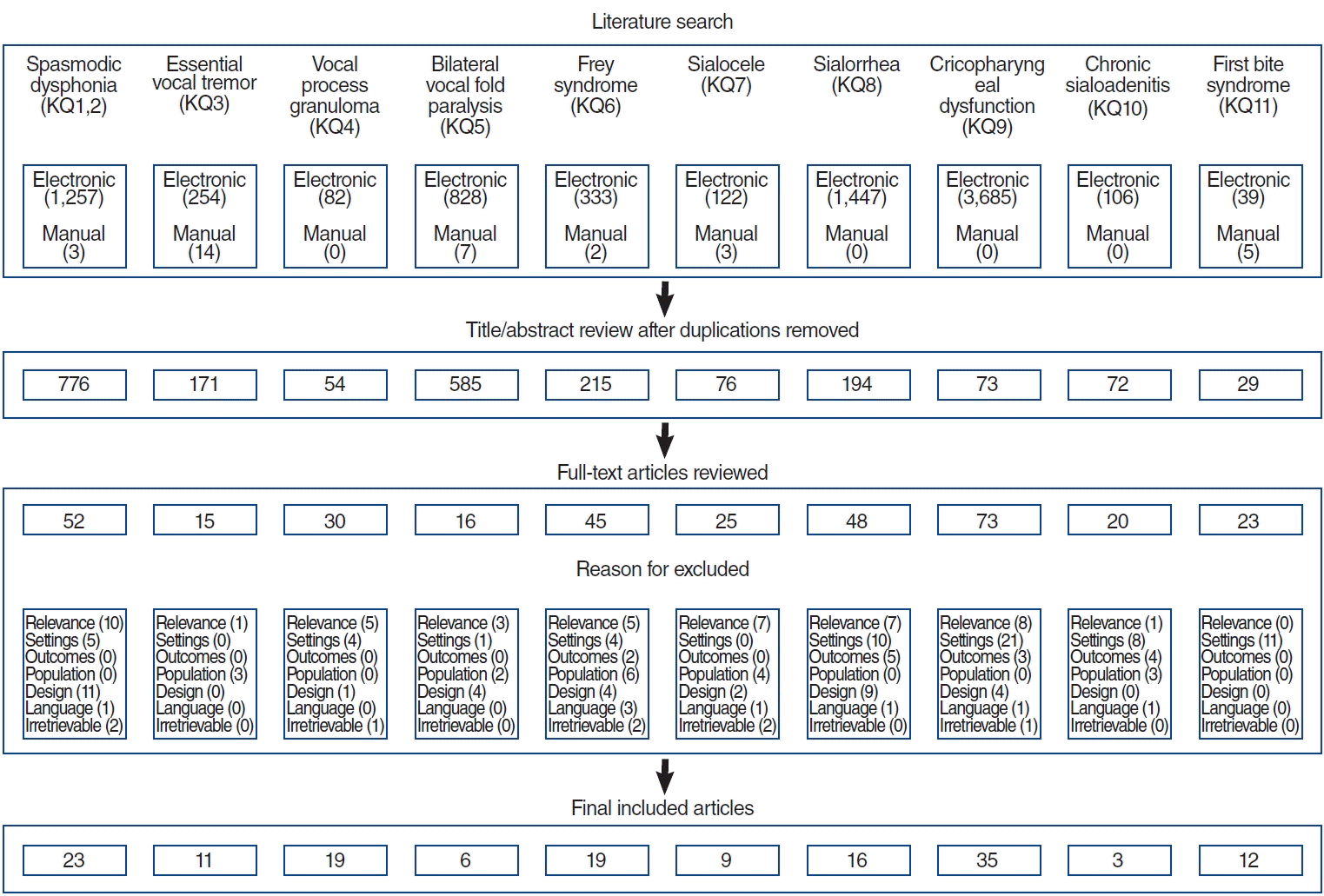
After determining the KQs, the committee identified search words for a systematic literature review. The databases used to search the literature included PubMed, Embase, the Cochrane Library, and KoreaMed. The managing and working group members performed the literature search on August 31, 2021. We collected all retrieved articles into Endnote X9.3 (Thomson Reuters). After removing duplicates, the committee members refined the database by excluding irrelevant papers after reading the titles and abstracts. Then, the committee members performed a fulltext review to determine the final references. The flowchart of included and excluded articles and the detailed search strategy are depicted in Fig. 1 and Supplementary Table 1 and 2, respectively.
Quality assessment of the literature, grades of recommendations, and evidence levels
We categorized the articles for developing each recommendation to determine the evidence level as follows: (1) randomized controlled trial (RCT) or well-conducted systematic review or meta-analysis, (2) prospective cohort study without randomization, (3) multi-center case-control study, (4) retrospective study, and (5) expert opinion or case series. For quality assessment, we used the Cochrane Risk of Bias tool for RCTs, the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS, version 1.5; Cochrane) for non-critical control studies (non-RCTs and observational studies), and a measurement tool to assess the methodological quality of systematic reviews for systematic reviews or meta-analyses [25,26].
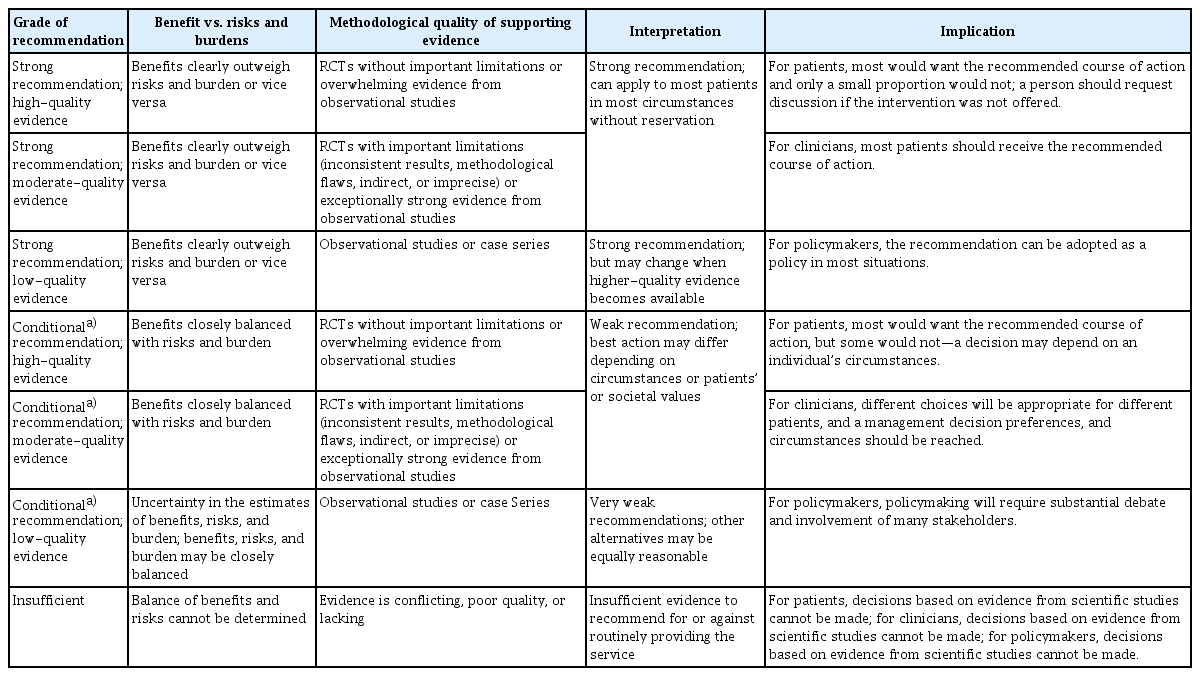
We divided the evidence level into high-, moderate-, and low-quality evidence [25]. After an in-depth discussion, we determined the strength of recommendations considering the evidence level, disease burden, risk/benefit of statements, and local medical circumstances. The American College of Physicians’ grading systems were adopted, consisting of two basic recommendation levels (“strong” and “conditional”) (Table 3).
Consensus regarding the recommendations and draft development
We conducted a Delphi survey to reach agreement on the recommendations for each KQ. We sent e-mails to physicians in the KSLPL with abundant clinical experience. The survey participants chose their opinion according to one of the following responses: fully agree, agree, neither agree nor disagree, disagree, or fully disagree. If more than two-thirds of the panel members responded with “fully agree” or “agree,” we decided to reach an agreement on each recommendation (Supplementary Table 3).
Plan for release and update of guidelines
This CPG will be published in an open-access journal in English. The guideline will be updated every 5 to 7 years to reflect new clinical data and the latest trends.
KQ 1. Is BT injection effective for improving voice quality in adductor spasmodic dysphonia (ADSD)?
Recommendation
(A) BT injection effectively improves voice quality and quality of life (QOL) in patients with ADSD. (Strong recommendation, High-quality evidence)
(B) Unilateral or bilateral BT injection into the thyroarytenoid (TA) muscles improves vocal quality for patients with ADSD. (Strong recommendation, Moderate-quality evidence)
SD is dystonia localized in the larynx, causing excessive involuntary contraction of the vocal folds. Depending on the affected laryngeal intrinsic muscles, SD is classified into adductor, abductor, and mixed types. The adductor type accounts for most cases of SD, while the abductor type accounts for only 10%–17% [27]. ADSD is characterized by a strained, strangulated voice and sudden voice interruption during phonation. It occurs due to an involuntary spasm of the TA muscle, resulting in abrupt glottal closure. In addition, it shows so-called “task specificity,” with worsened voice quality during the vocalization of voiced consonants. Abductor spasmodic dysphonia (ABSD) is caused by an involuntary spasm of the posterior cricoarytenoid (PCA) muscle acting at the opening of the vocal folds. These patients present with whispered, breathy, and weak vocalization.
The treatment of SD focuses on maintaining QOL by controlling the patient’s symptoms. The treatment strategy includes speech therapy, pharmacological therapy, BT injections, and surgery. BT injections are one of the most effective options. SD is a rare disease, making it difficult to conduct a large-scale randomized study [28]. However, decades of accumulated clinical experience have proven the effectiveness of BT for SD [29]. BT induces chemical denervation of the affected muscles and preserves vocal quality against involuntary contractions. Repeated BT injections into the vocal folds are considered the safest and most effective method.
Many studies have emphasized the effect of BT injections on patients with ADSD. Troung et al. [30] reported significant gains in evaluating the fundamental frequency, fundamental frequency range, spectrographic analysis, voice severity, and patient’s voice after BT injections in patients with ADSD. Recently, Hyodo et al. [31] demonstrated the efficacy and safety of BT injections in a multi-center, double-blind, controlled study of 22 patients with ADSD. Boutsen et al. [29] conducted a meta-analysis of 30 studies published from 1988 to 1999, demonstrating a significant improvement in voice quality with every evaluation method, including psychological, acoustic, auditory, and self-rating. Subgroup analyses showed that BT benefits voice quality regardless of the injection period (short-term vs. long-term) and injection site (unilateral vs. bilateral). Faham et al. [32] performed a meta-analysis on changes in QOL after BT injections for SD patients in 17 studies. They reported significant improvements in vocal quality after injections, measured by the Voice Handicap Index-30, Voice-Related QOL, and Voice Handicap Index-10. In their prospective study, Courey et al. [33] found that the perception of dysphonia significantly decreased, and social functioning and perception of mental health improved after injections of BT in patients with SD.
However, the injection site (unilateral vs. bilateral), interval, and dosage are difficult to standardize because of a lack of controlled studies for patients with SD [34]. A bilateral injection may augment clinical efficacy, but increase treatment-related complications, including breathy voice, aspiration, and dysphagia. In a retrospective study of 272 patients, Dharia and Bielamowicz [35] showed that bilateral injections were more effective in generating an optimal effect/side effect profile. However, in a randomized controlled study of 50 patients, unilateral and bilateral BT injections showed equal improvement in the symptoms of SD, and bilateral injections showed a longer duration of excessive phonatory airflow than unilateral injections [36]. In a study of 137 patients, Lee et al. [37] showed that unilateral injections had fewer side effects, but needed more frequent applications than bilateral injections. In a prospective study of 31 patients, Upile et al. [38] found that unilateral injections led to fewer voice complaints and lower complication rates than bilateral injections. Bielamowicz et al. [39] concluded that unilateral injections showed a better optimized and consistent treatment effect/side effect profile. Langeveld et al. [40] reported that the duration of voice improvement and dyspnea were not different between injection sites, but more patients had swallowing problems after bilateral injections. Koriwchak et al. [41] suggested that alternating unilateral injections may be helpful in patients who have a breathy voice after bilateral injections. Based on the results of these studies, unilateral injections may reduce the incidence of side effects while preserving clinical efficacy.
BT is applied with a flexible laryngoscope or under electromyographic guidance. Both injection methods have been shown to be effective. In an RCT, Kim et al. [42] observed no significant difference between injection methods in the duration of effect or treatment-related complications, including breathy voice and aspiration. Fulmer et al. [43] stated that the BT effect mostly depends on the surgeon’s experience, regardless of the injection method. Cha et al. [19] compared liquid BT with freezedried BT. They revealed that both groups exhibited improvements and found no significant difference in the impact duration between the two groups. The injection doses of BT reported in various studies ranged from 1.5 to 3.5 U. BT injection doses and intervals have not been standardized and may be adjusted according to the degree of symptom and treatment response. Rosow et al. [44] suggested that starting with a low amount of approximately 1.25 U on both sides at first and gradually increasing the dose might reduce side effects. Novakovic et al. [45] reported that an individually adjusted dose could maximize effects while reducing side effects in 133 patients with SD.
KQ 2. Is BT injection effective for voice improvement in patients with ABSD?
Recommendation
(A) BT injection effectively improves symptoms in patients with ABSD. (Conditional recommendation, Moderate-quality evidence)
(B) Alternating unilateral injections may reduce treatment-related airway obstruction in patients with ABSD. (Conditional recommendation, Low-quality evidence)
The PCA muscle is the only adductor laryngeal muscle and should be the target of BT injection for ABSD. In order to suppress abductor muscle spasms, physicians can choose two routes: inserting the needle in the posterior side of the larynx while rotating the thyroid cartilage to the opposite side, or directly through the cricothyroid membrane under laryngoscopic guidance. Because of the potential risk of airway compromise, BT injections for ABSD are commonly staged at intervals.
The efficacy of BT in controlling ABSD has been reported to be less consistent and variable in terms of improvement, compared to its substantial effectiveness in improving voice symptoms in nearly all patients with ADSD. Bielamowicz et al. [46] found limited benefits in their BT injection results for 15 patients with ABSD. Blitzer et al. [27] reported that 90% of patients with ADSD achieved normal voice compared to only 67% among patients with ABSD. The average duration of effect was longer, with 15.1 weeks in ABSD and 10.5 weeks in ABSD. They stated that only 20% of patients improved with an initial unilateral BT injection. The remainder required subsequent injections on the contralateral side to enhance their voice outcomes. Some authors reported that they could achieve successful voice outcomes with simultaneous bilateral injections. Woodson et al. [47] described asymmetric-dose injections of BT into the PCA muscle. They began with 5 U on the dominant side and 1.25 U on the non-dominant side as an initial dose. They conducted a step-wise increment protocol at 3-week intervals until optimal voice quality was reached. Klein et al. [48] reported an 89% improvement in symptoms after injecting BT on the same day in 14 patients with ABSD.
KQ 3. Is BT injection effective for voice improvement in patients with EVT?
Recommendation
Laryngeal BT injection may be helpful in improving voice quality for patients with EVT. (Conditional recommendation, Low-quality evidence)
EVT is a disease characterized by involuntary and regular movement of the vocal folds [49]. The main differential diagnostic point of EVT from SD is rhythmic tremors during phonation and rest. Approximately 60% of patients with essential tremor have EVT accompanied by other involuntary tremors, including the extrinsic laryngeal, pharyngeal, and palatal muscles [50,51]. Vocal symptoms may worsen during vowel pronunciation [51].
Pharmacological treatment for systemic tremors using propranolol or pyrimidone seems less effective in EVT. It may also cause systemic adverse effects, including fatigue, nausea, vomiting, and dizziness [52]. Thus, physicians prefer to use local treatment options to improve voice outcomes in those patients. The literature shows that BT injection is an effective treatment in EVT, with a 50%–65% subjective improvement rate, and the subjective improvement rate of BT injections is lower in EVT than in SD [53].
In a randomized controlled study comparing 15 U of BT and propranolol (80 mg/day), Guglielmino et al. [52] reported a significant voice improvement in BT injections. Hertegard et al. [54] reported substantial improvement in the fundamental frequency after BT injections in patients with EVT. They reported that 67% of EVT patients improved in voice perceptual evaluation. Kaye and Blitzer [55] showed tremor reduction in more than 70% of patients and voice improvement in 56%–100% of patients. Other reports showed consistent results in subjective or objective voice evaluations [56,57].
The motion vector of occurring tremors should be considered when selecting the injection site of BT in patients with EVT. For horizontal glottic tremors, the TA and lateral cricoarytenoid (LCA) muscles are the target injection point. The main target is the infrahyoid extrinsic laryngeal muscles, including the sternohyoid and sternothyroid muscles, for vertical glottic tremors [55]. The motion vector causing dominant symptoms is the first target for mixed tremors, but injection into all muscles causing tremors is considered if the symptoms do not improve by more than 50%. If motion vectors for the symptom expression level are similar, injection into the external laryngeal muscles is recommended first and then into the TA muscle 2 weeks later [57]. According to a comparative study by Orbelo et al. [58], clinicians tend to use lower doses for patients with EVT than for those with SD, with an average of 5.02±1.65 U and 6.80±2.79 U, respectively. When applied individually, the physician should adjust the injection dose according to the treatment response and adverse effects, including hoarseness and dysphagia [59]. Since there are still few reports in the literature on the effects of BT in essential tremors, additional studies on the appropriate dosage of BT are needed.
KQ 4. Is BT injection effective for improvement in patients with vocal process granuloma (VG)?
Recommendation
BT injection may be helpful for treating VG. (Conditional recommendation, Low-quality evidence)
VG results from chemical irritations caused by gastric acid or excessive physical contact due to overuse, habitual coughing, or endotracheal intubation [60]. Various treatment options exist, including reducing irritation (voice therapy, proton pump inhibitors, and BT), steroid injection, and laryngeal microsurgery [61-63]. Despite the existence of multiple treatments, VG is considered an intractable disease, with a reported recurrence rate of up to 90% [64].
BT treatment for VG was first reported in 1995 by Nasri et al. [4]. BT induces the paralysis of the vocal fold adductor muscles, thereby reducing excessive contact between two vocal processes. The TA muscles are considered the first injection site, but injection into the LCA or interarytenoid muscle is also considered [65-68]. The dose for a single injection varies from 1.5 to 30 U among reported studies [69,70]. Although it is generally preferable to administer BT under the guidance of a laryngoscope, some physicians opt for laryngeal electromyography. Ho et al. [71] achieved an 87.5% complete remission rate in VG with 1 U of BT using laryngeal electromyography.
The remission rate of VG after BT injections varies among studies [64,72]. Lee et al. [73] analyzed 590 patients with VG from 18 hospitals in Korea and compared the results according to treatment type. They found that BT had a superior therapeutic effect, with a remission rate of 74%. Other treatments had lower remission rates: simple observation had a 20.5% rate, steroid injection had a 31.6% rate, proton pump inhibitor administration had a 44.0% rate, and voice therapy had a 60% rate. However, this study was based on retrospective data collection, so the results should be interpreted with caution. BT injection could be considered as an alternative treatment option for persistent cases where other treatments have failed [73-77].
KQ 5. Is BT therapy effective for improving airway maintenance in BVFP?
Recommendation
BT injection may be helpful in improving airway maintenance for patients with BVFP who have mild airway distress. (Conditional recommendation, Low-quality evidence)
BVFP is a rare condition that arises from damage to the bilateral recurrent laryngeal nerves, which can be caused by trauma, tumor compression, invasion, or surgery. Patients with BVFP present with various symptoms, which correlate with the degree of the glottal gap during phonation. The symptoms encompass normal to severe breathy voice, dyspnea, and dysphagia. Patients with a minimal glottal gap during phonation may show only airway distress without subjective voice change.
The primary treatment goal for BVFP is to maintain the patient’s airway. For patients experiencing minor breathing difficulties, conservative treatments such as supplying oxygen may suffice. For those with more severe breathing difficulties, surgery to bypass the glottic obstruction and widen the glottis is recommended to secure the airway [78,79]. Tracheostomy, a common method for bypassing glottal obstruction [7], is a safe and straightforward procedure in emergency situations. However, long-term use of a tracheal tube can diminish the patient’s QOL and increase the risk of pressure necrosis and tracheal infection. Procedures that permanently widen the glottis (such as arytenoidectomy, cordectomy, or cordotomy) are not suitable for patients with mild to moderate dyspnea or an uncertain prognosis for neural function recovery [7]. BT injections offer an alternative for securing the airway in patients with mild to moderate airway distress. They are most suitable for patients who experience dyspnea upon exertion and have little to no discomfort. The benefit of BT injections is their reversibility, which allows destructive surgery to be avoided and provides time for potential neural function recovery.
The TA or LCA muscle is the main target for BVFP, with injections administered unilaterally or bilaterally. The injection diminishes the vocal fold adduction force, reduces the vocal fold tone, and strengthens the vocal fold adductor muscle (the PCA muscle). BT also affects synkinesis caused by abnormal reinnervation in the vocal fold adductor during recovery. Specifically, BT blocks the unwanted adduction of neural activities during inspiration, stabilizing inspiration. BT is considered a temporary tool, with its effects lasting approximately three months after injection. However, repeated injections of BT induce TA muscle atrophy, with an impact on securing the airway [80].
The treating physician should start with a minimum dose for BVFP because BT aimed at improving airway symptoms could potentially exacerbate the quality of vocalization and swallowing [81]. The physician should then adjust the injection doses while carefully monitoring the patient’s symptoms. For instance, Zealear et al. [82] administered a minimum dose of 2.5 U of BT bilaterally into each vocal fold.
KQ 6. Is BT therapy effective for improving symptoms of patients with Frey’s syndrome?
Recommendation
Intradermal BT injection effectively improves symptoms of Frey’s syndrome. (Conditional recommendation, Low-quality evidence)
Intradermal BT injection effectively improves symptoms of Frey’s syndrome. (Conditional recommendation, Low-quality evidence)
Frey’s syndrome, first described by Lucie Frey in 1923, involves gustatory sweating after trauma or surgery in the parotid region. The symptoms of Frey’s syndrome include sweating, flushing, tingling, heat, and pain in the parotid region in response to eating or chewing food [83]. Frey’s syndrome is a significant concern both immediately after parotidectomy and even up to 5 years postoperatively [84]. The underlying mechanism involves the abnormal reinnervation of the parasympathetic nerve into the sweat glands and blood vessels of the denervated skin. Initially thought to be rare, it is now estimated that approximately 7%– 30% of patients exhibit overt clinical symptoms after parotidectomy [84-86]. The diagnosis of Frey’s syndrome can be confirmed through Minor’s iodine-starch test, but physicians can often make a diagnosis based on the patient’s clinical history. Neumann et al. [85] reported that approximately 60% of patients tested positive for the syndrome after parotidectomy, while 23% of patients reported symptoms causing discomfort in their daily lives. Frey’s syndrome has significant psychological and social impacts, which prompted the development of various surgical methods to prevent it [87]. The fundamental surgical principle involves strengthening or establishing a barrier between the parasympathetic nerve and parotid parenchyma, as well as the overlying subcutaneous tissue [88-90].
Two medical approach for managing Frey’s syndrome post-surgery are the use of anticholinergic drugs and BT injections. However, the effects of anticholinergic drugs are somewhat limited [91]. Drobik and Laskawi [5] first reported the successful treatment of Frey’s syndrome with an intradermal injection of BT in 1995. Since then, intradermal BT injections have demonstrated effective control over gustatory sweating in patients with Frey’s syndrome (as reviewed in [92]). The reported duration of action for intradermal BT injections varies across studies, ranging from roughly 3 months to 17.3 months. However, it appears to have a longer effect than BT injected into the neuromuscular junction [93-95]. Repeated injections may enhance both the potency and the duration of the effective periods. The mechanism underlying this phenomenon is thought to be related to the degeneration and atrophy of the synkinetic nerve and the overlying sweat gland, caused by the continuous blockage of nerve endings.
According to a report by Laccourreye et al. [96], patients experiencing recurrent gustatory sweating perceive their condition as less severe compared to their initial severity. It is suggested that repeated injections may alleviate their symptoms. de Bree et al. [97] have also reported that the therapeutic effect tends to last longer following a second injection [98]. The typical distance between injection sites is 10–20 mm. Each injection dose can range from 2.0 to 5.0 U, but the total dosage should not exceed 380 U [99]. Complications from BT injections are relatively rare. The most common complication is temporary paralysis of the muscles adjacent to the injection site, with symptoms usually resolving within three months [100].
KQ 7. Is BT therapy effective for improving symptoms of patients with sialocele?
Recommendation
Physicians may consider BT injection for patients with sialocele in whom conservative treatment has failed. (Conditional recommendation, Low-quality evidence)
Sialocele mainly occurs as a complication of salivary gland surgery. A recent meta-analysis by Maharaj et al. [101] identified that 67% of all sialoceles were due to salivary gland surgery. In a multi-center study, Lee et al. [102] described that sialocele occurred in 6.4% of patients undergoing parotid surgery. It also occurs due to trauma or head and neck surgery [103-105]. Patients with sialoceles present with painless swelling, but sialocele can be complicated by fistula formation, resulting in infection.
Various conservative and surgical treatments have been proposed for managing sialoceles. Conservative treatments encompass repeated aspiration coupled with compressive dressing, pharmacologic therapy utilizing salivary gland inhibitors, and BT injection. However, surgical treatments may involve the reconstruction of damaged salivary gland ducts, ligation of these ducts to induce salivary gland atrophy, or complete sialadenectomy.
BT injection is the last conservative option for patients in whom other treatments have failed. BT controls sialocele by inhibiting saliva production in the gland. Vargas et al. [8] reported the first successful BT injection in four patients with sialoceles after partial parotidectomy in 2000. A prospective study conducted by Lee et al. [106] reported the effective use of BT injections in the surgical field of partial parotidectomy to reduce the incidence of sialocele. Laskawi et al. [107] suggested that BT, as the initial treatment, increases the success rate of fistula closure by approximately 90%. The dose of a BT injection is approximately 10–200 U per patient, with a reported success rate of approximately 70%–100% [101]. Even if there is no response after the first treatment, improvement after repeated treatment may be expected [101]. Send et al. [108] recommended using different doses according to the size of the fistula, stating that 20 U would be sufficient for small fistulas, but up to 50 U may be required for controlling large fistulas.
KQ 8. Is BT injection effective for the treatment of patients with sialorrhea?
Recommendation
BT injection may be helpful for treating VG. (Conditional recommendation, Low-quality evidence)
Drooling is a common occurrence in babies and early childhood, but it is considered pathological when it persists in adults. The primary causes of sialorrhea encompass central nervous system diseases such as Parkinson’s disease, cerebral palsy, and amyotrophic lateral sclerosis, infections, medication side effects, and damage to the marginal mandibular branch of the facial nerve during salivary gland surgery. Sialorrhea can diminish QOL as it complicates personal hygiene and hinders daily activities. This is particularly true for patients with neurological disorders or the elderly, as it can heighten the risk of infections, nutritional imbalance, and respiratory diseases [109-115]. The para-autonomic nervous system regulates salivation through the release of acetylcholine, which promotes salivation. BT works to inhibit the release of acetylcholine, thereby preventing excessive salivation. Multiple studies, including systematic reviews, meta-analyses, and randomized trials, have demonstrated the effectiveness of BT in treating sialorrhea [116-118].
Currently, BT-A is commonly used to treat sialorrhea. Although there are no standardized guidelines regarding the optimal therapeutic dose of BT-A, a review of existing literature suggests the following dosages: 5–30 U for injections into the submandibular gland and 5–75 U for injections into the parotid gland. However, doses between 5–10 U often prove ineffective for the parotid gland, necessitating doses between 30–200 U. These doses can be adjusted according to the patient’s weight and age [112,119-121]. Lungren et al. [122] proposed dosages based on patient weight: 15 U/gland for patients weighing less than 15 kg, 20 U/gland for patients between 15 to 25 kg, and 25 U/gland for those over 25 kg. Gonzalez-L et al. [119] conducted a study on patients with cerebral palsy aged 18 years or older, administering two injections of BT-A at a dose of 100 U, with follow-up assessments every 8 weeks. The treatment group showed significant symptom improvement compared to the untreated group, with reductions of 30% or more in total flow, and no significant differences in adverse events. Jost et al. [120] used doses of 75 U and 100 U of BT-A with follow-up intervals exceeding 64 weeks, demonstrating the long-term effectiveness and safety of the treatment. BT is directly injected into the submandibular and parotid glands, either on one side or both [115,123,124]. Ultrasound-guided injections ensure high accuracy. Wu et al. [112] reported that this method allows for the administration of the dose while observing the salivary gland or its surrounding areas, thereby minimizing potential adverse side effects, such as nerve damage.
The therapeutic effect persists for 3–6 months following injection, with peak efficacy observed between 1 to 8 weeks post-injection [115,119-121,123-128]. In a prospective, double-blind trial, Chinnapongse et al. [123] found that the therapeutic effect for Parkinson’s disease patients with sialorrhea could last up to 20 weeks or more. Isaacson et al. [124], in their RCT, reported a reduction in sialorrhea 1 week after BT injections, with this effect lasting for at least 13 weeks. Given the lack of universal guidelines regarding the number and intervals of injections, it is crucial to determine the need for repeated BT injections based on subjective improvement, objective symptom severity measures, and any adverse effects. Side effects are rare, but caution should be exercised for potential issues such as infection, trismus, xerostomia, dental cavities, dysgeusia, dizziness, sleep disorders, vision problems, and confusion [123,124]. Wu et al. [112] noted the occurrence of dysphagia at injection doses of 30–50 U, thick saliva secretion at 80–100 U, and sialadenitis and high treatment dropout rates at doses of 100–140 U.
While there are numerous reports on the treatment outcomes of using BT to manage sialorrhea, there is a lack of large-scale studies specifically focused on this condition. Consequently, detailed guidelines for the use of BT are not readily available. However, a thorough analysis of the literature suggests that precise injections of optimal doses can improve symptom management and minimize the occurrence of side effects and complications.
KQ 9. Is BT therapy effective for improving symptoms of CP dysfunction?
Recommendation
BT injection is an effective method for treating dysphagia caused by CP dysfunction. (Conditional recommendation, Low-quality evidence)
The upper esophageal sphincter (UES) consists of the CP and the thyropharyngeus muscle. The CP muscle plays a dominant role in its function. The UES opening is controlled by swallowing effort or intraluminal pressure. In the resting state, it is closed by a continuous contraction signal from the vagus nerve to prevent reflux. In cases of abnormal narrowing caused by achalasia, stenosis, or UES hypertrophy, dysphagia and aspiration occur [129]. Neurological diseases are common causes of CP dysfunction. These include cerebrovascular accidents, amyotrophic lateral sclerosis, myasthenia gravis, multiple sclerosis, and Parkinson’s disease. Additionally, tumorous conditions in the head and neck, fibrosis resulting from post-treatment sequelae, or Zenker’s diverticulum can also lead to CP dysfunction [130].
Various treatment methods have been introduced for CP dysfunction. Currently, endoscopic treatment is the primary approach, encompassing BT injection, myotomy, and dilatation using a balloon or bougies on the CP [131,132]. A recent scoping review paper by Dewan et al. [133] revealed that BT injections were the most favored method, accounting for 40% of all reports. This was followed by endoscopic CP myotomy (30%), dilatation (25%), and open CP myotomy (15%).
For patients with dysphagia associated with neuromuscular diseases, treatment for swallowing difficulties should be carried out concurrently with treatment for the underlying conditions. A video fluoroscopic swallowing study or manometry can be used in the baseline evaluation to diagnose CP dysfunction. If CP dysfunction is detected, the aforementioned treatment should be implemented alongside rehabilitation that focuses on swallowing therapy [134,135]. In 1994, Schneider et al. [3] were the first to administer BT injections to two patients with CP dysfunction. They reported that the BT injections effectively resolved the patients’ dysphagia without causing significant complications.
Since then, many physicians have attempted to treat CP dysfunction using BT injections. However, these studies inherently have limitations, as they were conducted on a small sample size of fewer than ten patients. These studies reported a wide range of success rates, from 20 to 100% [136-145]. Kelly et al. [146] conducted a retrospective analysis on a relatively larger sample of 49 patients. Their findings indicated that approximately 65% of patients experienced improved dysphagia-related outcomes following BT injections.
Well-designed studies comparing the effects of BT injections with those of other treatments are limited, as a Cochrane Database Review released in 2014 concluded that determining treatment homogeneity would be impossible [147]. In a systematic review conducted in 2016, Kocdor et al. [148] found that the overall success rate was 69% for BT injections, 73% for endoscopic dilatation, and 78% for CP myotomy. When comparing treatment success rates by etiology, they found that 78.6% of patients with neurological causes and 65% of patients who underwent treatment for head and neck cancer showed improvement in dysphagia-related symptoms following BT injections [147,149-154].
There are two injection methods: injection with direct vision under the guidance of a suspension laryngoscope or endoscope, and indirect injection under monitoring via guidance from electromyography, fluoroscopy, or computed tomography [155-159]. The BT injection dose varies from 2.5 U to 300 U, although the most commonly used doses in clinical practice fall between 50 and 100 U [136]. BT injections are not recommended for patients with CP fibrosis or stenosis. Instead, they are most suitable for patients experiencing CP spasms or those who have difficulty relaxing [160]. However, restricting the use of BT injections to these conditions is not necessary, as significant improvements in dysphagia have been observed in patients with weakened CP muscle tone [161]. While the risks associated with BT injections are typically minimal, their effectiveness tends to be short-lived, lasting approximately 4–7 months. This necessitates repeated administration, which can be seen as a drawback [162].
KQ 10. Is BT injection effective in reducing salivary gland origin pain in patients with chronic sialadenitis?
Recommendation
BT injection may be helpful in improving recurrent swelling and pain of the salivary glands in patients with chronic sialadenitis. (Conditional recommendation, Moderate-quality evidence)
To the best of our knowledge, there are currently no RCTs on the efficacy of BT injections in alleviating symptoms associated with chronic sialadenitis. A multi-institutional study conducted by Kwon et al. [163] examined the impact of BT injections on reducing pain and swelling in the salivary glands of 14 patients suffering from chronic sialadenitis. Their findings indicated that a single BT injection could improve symptoms such as pain and swelling, with no changes in the volume or function of the salivary glands observed after injection. In a study involving six patients with recurrent parotitis, Capaccio et al. [164] found that BT injections significantly reduced recurrent parotid swelling in all instances. A systematic review by Strohl et al. [9], which included 11 studies, found that 34 out of 35 patients reported either complete or partial improvement in recurrent sialadenitis symptoms. Moreover, 93% of 44 patients, inclusive of their case series, experienced symptom improvement following BT injections. It is also worth noting that the majority of the patients studied were those who continued to experience symptoms despite undergoing medical treatment or sialendoscopy prior to receiving the BT injection. This suggests that BT injections may offer superior symptom resolution.
The mean equivalent dosage of BT injections for alleviating symptoms of chronic sialadenitis was 60 U, with a range of 22.5– 100 U. The average number of injections administered was 1.8, ranging from 1 to 9 injections [9,164]. According to the study, most cases did not experience any side effects, although 12% of patients did develop dry mouth. There were no instances of facial paralysis reported. In a separate study conducted by Kwon et al. [163], the average dosage of a BT injection was 25 U per gland. Two patients (14%) reported treatment-related complications, each experiencing temporary dysphagia and dry mouth.
KQ 11. Is BT injection effective in reducing origin pain in patients with first bite syndrome?
Recommendation
Physicians may consider BT injection for patients with first bite syndrome in whom conventional medical treatment has failed or as an initial treatment to control parotid area pain. (Conditional recommendation, Low-quality evidence)
First bite syndrome refers to an intensely sharp or cramping pain in the parotid gland region that occurs with the first bite of each meal and typically subsides with subsequent bites. The main mechanism seems to be an imbalance of sympathetic/parasympathetic innervation of the parotid gland [165]. Common etiologies include parotid gland or parapharyngeal space surgery, with a reported incidence of up to 18% following these operations [166]. However, there are also reported cases where underlying tumors in the region were identified as the cause [167,168]. BT injections can reduce the cross-stimulatory contraction of intraparotid myoepithelial cells by blocking the parasympathetic neurotransmitters.
Reports on BT injection therapy for first bite syndrome are limited due to its rarity. The majority of studies are either individual case reports or case series [11,169-171]. Lee et al. [11] documented their first successful case series using BT injections to manage symptoms of first bite syndrome. They administered a total dose of 33 U of BT, divided into three doses, in the most painful areas of five patients. According to a literature review by Laccourreye et al. [172], BT injections consistently improved the symptoms of first bite syndrome. Persaud et al. [173] reviewed two studies and reported that the therapy effectively reduced pain and improved QOL in all six patients with first bite syndrome. Steel and Robertson [174] reviewed eight studies and found that 20 out of 24 patients experienced partial or complete pain relief following an intraparotid injection of BT.
In most cases, BT was injected several times into the most painful zone in the parotid gland, and the total injection dose per treatment session ranged between 22.5 and 75 U. Patients who received relatively small amounts of BT injections either experienced partial symptom relief or recurrence after 4–6 months. There were no reported incidents of complications other than mild discomfort during the injection. The authors also noted that more patients showed therapeutic responses following BT injections than after the administration of neuropathic drugs, such as carbamazepine, gabapentin, pregabalin, amitriptyline, and clomipramine. The treatment effects of surgical therapy, such as remnant parotidectomy or tympanic neurectomy, are limited and pose a risk of surgical complications, including facial nerve injury [174,175].
SUMMARY
BT is safely and effectively used in otolaryngology to treat various diseases. In this study, the committee produced evidence-based BT treatment guidelines to help clinicians make safe and effective treatment decisions for 10 diseases (SD, EVT, vocal fold granuloma, BVFP, Frey’s syndrome, sialocele, sialorrhea, CP dysfunction, chronic sialadenitis, and first bite syndrome). Among the 13 recommendations, our committee presented strong recommendations for ADSD and sialorrhea. High-quality evidence was found for the use of BT injection to treat patients with ADSD. The searched literature showed that BT injection has a high probability of success in improving symptoms for the target diseases, but additional research is needed to improve the level of evidence.
HIGHLIGHTS
▪ The Korean Society of Laryngology, Phoniatrics and Logopedics created a task force to establish clinical practice guidelines for the use of botulinum toxin (BT) in otolaryngology.
▪ The committee reported 13 final recommendations with detailed evidence profiles.
▪ The guidelines primarily target all clinicians applying BT to the head and neck area.
▪ The guidelines aim to promote an improved understanding of the safe and effective use of BT by policymakers and counselors, as well as patients scheduled to receive BT injections.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors. Data curation: all authors. Formal analysis: all authors. Methodology: CHR, MJB, SWL. Project administration: CHR, MJB, SHC, SWL. Visualization: CHR, MJB, SWL. Writing–original document: all authors. Writing–review & editing: CHR, MJB, JHW, SWL.
Acknowledgements
We gratefully acknowledge the support of Gi-Sung Yoon, a medical librarian at Soonchunhyang University.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found online at https://doi.org/10.21053/ceo.2023.00458.
Search strategy for the use of botulinum toxin in otolaryngology field (performed on Aug 31, 2021)
Search strategy along the key question (KQ)
Delphi questionnaire for the use botulinum toxin in otolaryngology field